* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Interaction of Antiparallel Microtubules in the
Tissue engineering wikipedia , lookup
Microtubule wikipedia , lookup
Endomembrane system wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
List of types of proteins wikipedia , lookup
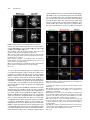
The Plant Cell, Vol. 23: 2909–2923, August 2011, www.plantcell.org ã 2011 American Society of Plant Biologists. All rights reserved. Interaction of Antiparallel Microtubules in the Phragmoplast Is Mediated by the Microtubule-Associated Protein MAP65-3 in Arabidopsis W Chin-Min Kimmy Ho,a Takashi Hotta,a Fengli Guo,a,1 Robert W. Roberson,b Yuh-Ru Julie Lee,a and Bo Liua,2 a Department b School of Plant Biology, University of California, Davis, CA 95616 of Life Sciences, Arizona State University, Tempe, AZ 85287 In plant cells, microtubules (MTs) in the cytokinetic apparatus phragmoplast exhibit an antiparallel array and transport Golgi-derived vesicles toward MT plus ends located at or near the division site. By transmission electron microscopy, we observed that certain antiparallel phragmoplast MTs overlapped and were bridged by electron-dense materials in Arabidopsis thaliana. Robust MT polymerization, reported by fluorescently tagged End Binding1c (EB1c), took place in the phragmoplast midline. The engagement of antiparallel MTs in the central spindle and phragmoplast was largely abolished in mutant cells lacking the MT-associated protein, MAP65-3. We found that endogenous MAP65-3 was selectively detected on the middle segments of the central spindle MTs at late anaphase. When MTs exhibited a bipolar appearance with their plus ends placed in the middle, MAP65-3 exclusively decorated the phragmoplast midline. A bacterially expressed MAP65-3 protein was able to establish the interdigitation of MTs in vitro. MAP65-3 interacted with antiparallel microtubules before motor Kinesin-12 did during the establishment of the phragmoplast MT array. Thus, MAP65-3 selectively crosslinked interdigitating MTs (IMTs) to allow antiparallel MTs to be closely engaged in the phragmoplast. Although the presence of IMTs was not essential for vesicle trafficking, they were required for the phragmoplast-specific motors Kinesin-12 and Phragmoplast-Associated Kinesin-Related Protein2 to interact with MT plus ends. In conclusion, we suggest that the phragmoplast contains IMTs and highly dynamic noninterdigitating MTs, which work in concert to bring about cytokinesis in plant cells. Introduction In plant cells, cytokinesis is brought about by the phragmoplast, which assembles the cell plate upon the completion of nuclear division. In the phragmoplast, microtubules (MTs) are organized in an antiparallel array and are aligned perpendicularly to the division plane. The antiparallel MTs have their plus ends located at or near the cell division site and their minus ends facing two reforming daughter nuclei. The organization of such a bipolar MT array allows directional transport of Golgi-derived vesicles toward the division site (Staehelin and Hepler, 1996; Jürgens, 2005). The array is a result of polymerization/depolymerization and reorganization of MTs with mixed polarities in the central spindle (Zhang et al., 1990). Whereas the overall organization of phragmoplast MTs is well appreciated, the method by which they are organized into such an elegant array while remaining highly dynamic remains unknown. In Haemanthus endosperm cells, short cross-bridges were detected between antiparallel MTs from opposite sides of 1 Current address: Stowers Institute for Medical Research, Kansas City, MO 64110. 2 Address correspondence to [email protected]. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Bo Liu (bliu@uc davis.edu). W Online version contains Web-only data. www.plantcell.org/cgi/doi/10.1105/tpc.110.078204 the phragmoplast (Hepler and Jackson, 1968; Euteneuer and McIntosh, 1980). In the mature phragmoplast, an electron opaque zone of ;0.3 mm was observed by transmission electron microscopy (TEM), where an overlap of MTs from both sides of the developing cell plate can be seen (Hepler and Jackson, 1968). Such a MT interdigitation phenomenon has also been observed in the phragmoplast in the moss Physcomitrella patens (Hiwatashi et al., 2008). In dividing somatic cells of Arabidopsis thaliana prepared by rapid freezing/freeze substitution, however, the majority of phragmoplast MTs do not seem to overlap when observed by electron tomography (Austin et al., 2005). It was thus suggested that a cell plate assembly matrix (CPAM) was responsible for engaging the antiparallel MTs in the phragmoplast. This observation contradicts the conventional view of how antiparallel MTs are held together in the phragmoplast. However, it is in agreement with immunofluorescence images of antitubulin and images of green fluorescent protein (GFP)– marked MTs in somatic cells, because the corresponding images show a dark line in the middle of the phragmoplast (Liu et al., 1995; Yoneda et al., 2005). Phragmoplast MTs undergo rapid turnover (Hush et al., 1994). Using antibody immunofluorescence, it has been shown that the MT-nucleating factor g-tubulin preferentially decorates MTs toward the distal ends, facing the daughter nuclei (Liu et al., 1994; Liu et al., 1995). This suggests that MTs are nucleated from the distal ends and polymerized toward the division site. However, in permeabilized tobacco (Nicotiana tabacum) cells and 2910 The Plant Cell Haemanthus endosperm cells, exogenous tubulins were incorporated into the phragmoplast overwhelmingly in its midline (Vantard et al., 1990; Asada et al., 1991). These studies also showed that newly polymerized MTs overlapped and then slid outward. The presence of the MT plus end-tracking protein End Binding1 (EB1) in the phragmoplast midline also favors the notion that MT nucleation takes place in the phragmoplast midline (Chan et al., 2005; Bisgrove et al., 2008; Komaki et al., 2010). Thus, further examination with high spatial and temporal resolution is necessary to characterize MT nucleation events in live phragmoplasts. In the past few years, the list of proteins known to decorate the phragmoplast midline has grown (Otegui et al., 2005). Among them, the most likely MT cross-linking factors are proteins in the MT-associated protein65 (MAP65)/Anaphase Spindle Elongation1/Protein Regulator of Cytokinesis1 family and members of the Kinesin-12 subfamily (Jiang and Sonobe, 1993; Lee and Liu, 2000; Smertenko et al., 2000; Pan et al., 2004; Smertenko et al., 2008). MAP65 proteins can form a homodimer to promote antiparallel MT bundling in the phragmoplast and in other MT arrays during cell division (Smertenko et al., 2004; Gaillard et al., 2008). Thus, they can bridge overlapping MTs in the phragmoplast. Similar bridging effects have also been proposed and observed in fungal and animal cells (Zhu et al., 2006; Kapitein et al., 2008). Among the nine MAP65 proteins in A. thaliana, MAP65-3 plays a primary role in cytokinesis, as its loss leads to a wider dark central gap in the phragmoplast by tubulin immunofluorescence and frequent failures in cytokinesis (Müller et al., 2004; Caillaud et al., 2008). However, MAP65-3 is not required for the bipolar appearance of phragmoplast MTs. Nevertheless, this result suggests that MT segments near their plus ends are likely bundled by MAP65-3 in the phragmoplast, and such a bundling phenomenon is critical for cell plate formation. In contrast to MAP65, Kinesin-12 motors seem to play a role in keeping MT plus ends anchored at the division site, because, in their absence, MTs cross over the phragmoplast midline and become continuous bundles (Lee et al., 2007). Consequently, proteins like the syntaxin KNOLLE, normally localizing to the phragmoplast midline, become mis-localized in the Kinesin-12 mutant because of the disorganization of MT plus ends. We sought to establish how the plus ends of antiparallel MTs are engaged with each other in the phragmoplast and how the activities of proteins involved in cytokinesis are integrated at the site of cell division. The results reported here show that a subpopulation of spindle midzone and phragmoplast MTs are bundled by MAP65-3 in an antiparallel manner. Meanwhile, other MTs in the region do not overlap. We conclude that the integration of MT plus ends by MAP65-3 is required for kinesin motors to interact with the plus ends, but is not essential for vesicle trafficking during cell plate assembly. Results MT Interdigitation in the Phragmoplast Detected by TEM To investigate MT organization in the phragmoplast midzone, root meristematic cells of A. thaliana were prepared by highpressure rapid freezing and freeze substitution for TEM obser- vation. At an early stage of cytokinesis, when Golgi-derived vesicles began to accumulate at the division site, long MTs were frequently detected in the phragmoplast formed between two reforming daughter nuclei (Figure 1A). Although many MTs were terminated in regions where vesicles accumulated, others crossed the midline and overlapped (Figures 1A and 1B). Such a MT-overlapping phenomenon was also found at the later stages of cytokinesis, when a complex tubular–vesicular network was already established in the middle of the phragmoplast (Figure 1C). MTs were found to pass over the phragmoplast midline (Figure 1C). In cells exhibiting a fenestrated early cell plate, MTs were seen to cross the open region left by membrane sacs (Figures 1D and 1E). Again, MTs from one side were found to overlap with those coming from the opposite side of the cell (Figures 1D and 1E). Electron-dense materials were detected between the overlapping MTs as if they were bridges (arrow, Figure 1E). We found that a subpopulation of antiparallel MTs penetrated the vesicle-dense region and sometimes overlapped with each other in the phragmoplast at different stages of cytokinesis, whereas many MTs ended before reaching the cell plate. Analysis of MT Plus Ends in the Phragmoplast Published data using fixed samples suggest that antiparallel MTs in the phragmoplast are separated by the CPAM (Austin et al., 2005). Previously, the EB1c protein was detected in the phragmoplast by immunofluorescence, and its signal was pronounced near the midline (Bisgrove et al., 2008). To examine the activity of MT plus ends in the phragmoplast of live cells, we observed cells expressing fluorescently labeled EB1c using a transgenic line in which an EB1c-33FLAG-GFP fusion protein was expressed under the control of the native EB1c promoter. The transgene completely suppressed the oryzalin hypersensitive phenotype of the eb1c mutant (see Supplemental Figure 1 online). In cells undergoing cytokinesis, EB1c-33FLAG-GFP signals were detected in the phragmoplast (Figure 2). The signal was widely present in the phragmoplast at the early stages of cytokinesis, and numerous EB1c-33FLAG-GFP comets were found to move toward the division site (see Supplemental Movie 1 online). At the later stages of cytokinesis, EB1c-33FLAG-GFP became gradually restricted to the midline of the phragmoplast, where the cell plate was going to form (Figure 2). If antiparallel MTs were spaced by the CPAM, we would expect to see a persistent dark line in the middle of the developing phragmoplast. In all cytokinetic cells examined, no dark line was detected at any stage of the phragmoplast. This finding suggests that it is unlikely that the developing cell plate prevents MT polymerization from taking place at the division site, and that the plus ends of polymerizing, stabilized, or paused MTs likely overlap in the middle region of the phragmoplast. MAP65-3 Is Required for the Engagement of Antiparallel MTs in the Phragmoplast An earlier study showed that the ple mutation in the gene encoding MAP65-3 causes the widening of the dark gap in the middle of the phragmoplast when MTs were revealed by antitubulin immunofluorescence (Müller et al., 2004). A plausible Phragmoplast Microtubule Organization 2911 somes were completely segregated in the wild-type cells, MTs in the central spindle became prominent and aligned in parallel without an obvious break in the middle (Figure 3A; 100%, n = 37). This phenomenon suggested that the MTs initiated from the opposite regions near segregated chromosomes were bundled together and tightly engaged. In a dyc283 mutant cell at a similar stage, however, MTs became splayed away from the segregated chromosomes (Figure 3B; 91%, n = 66). The two populations of MTs did not show any sign of interaction in this mutant cell. Later, parallel bundles of phragmoplast MTs were aligned perpendicularly to the division plane, and left a sharp dark line in the middle of the wild-type phragmoplast, as shown by antitubulin immunofluorescence (Figure 3C; 100%, n = 155). In mutant cells at similar stages, bundles of phragmoplast MTs tilted away from the right angle relative to the division plane (Figure 3D; 100%, n = 280). Such a MT alignment pattern in dyc283 cells indicated that the antiparallel MTs in the phragmoplast became disengaged. Endogenous MAP65-3 Highlights Overlapping MTs during Late Anaphase and Cytokinesis Because of the altered pattern of antiparallel MTs in the dyc283 mutant cells, we wanted to dissect how endogenous MAP65-3 interacted with MTs during the anaphase–telophase transition. Antibodies prepared against a truncated fusion protein expressed in bacteria were shown to recognize a single band Figure 1. Interaction between Antiparallel MTs in the Phragmoplast Observed by TEM. (A) Phragmoplast at an early stage of cytokinesis. Most MTs do not pass through the division site enriched with vesicles (arrow). MTs crossing the phragmoplast midline are indicated by arrowheads. Two reforming daughter nuclei are marked by asterisks. (B) A region of the early phragmoplast is enlarged to show MTs crossing the midline (arrowheads). (C) A mature phragmoplast. The tubular–vesicular network is greatly elaborated at the division site (arrow). Examples of MTs crossing the midline are indicated by arrowheads. (D) Clustered MTs between the membranous sacks (asterisk). MTs from two sides of the forming cell plate are closely engaged. (E) Interdigitation of MT clusters in regions flanked by membranous sack (asterisks, *). Electron-dense materials (arrow) are visible in the MToverlapping region. Bar in (A) = 0.5 mm; bar in (B) to (E) = 0.2 mm. explanation could be that the antiparallel MTs might interact with each other at their plus ends in the absence of MAP65-3. To investigate how MT plus ends behaved during the development of the phragmoplast, the interface of antiparallel MTs was examined in the dyc283 mutant, which contains an inactivating insertion allelic to ple (Caillaud et al., 2008). After sister chromo- Figure 2. EB1c Is Enriched in the Phragmoplast Midline. Time-lapsed images show the changes in fluorescence of the EB1c33FLAG-GFP fusion in two cytokinetic cells. At the 0-s time point, the cell on the right has EB1c signals across the entire region of the phragmoplast (asterisk), whereas other more discrete comets can be found between the reforming daughter nuclei and the phragmoplast (arrows). The EB1c signal becomes more concentrated at the phragmoplast midline at later stages of division (arrowheads). This site coincides with that of the forming cell plate (arrowheads) as seen in the brightfield DIC image on the bottom right. Bar = 5 mm. 2912 The Plant Cell central spindle MTs became more prominently bundled (Figure 4E). MAP65-3 was associated with segments of interzonal MTs across the middle region of the central spindle (Figure 4D). It was also detected toward the distal ends of MTs emanating from spindle poles (Figure 4F), albeit with much lower signal intensity compared with the signal along bundled MTs in the central spindle. Upon further coalescence of interzonal MTs, the central spindle had two clear sets of MTs, and the dark Figure 3. Failed Engagement of Antiparallel MTs in dyc283 Cells. MTs are revealed by antitubulin immunofluorescence at late anaphase/ telophase ([A] and [B]) and cytokinesis ([C] and [D]) in the wild-type ([A] and [C]) and mutant ([B] and [D]) cells. (A) In a wild-type cell at late anaphase/telophase, MTs in the central spindle are highly bundled and aligned in parallel (arrows). (B) In a dyc283 cell, MTs nucleated from the regions of sister chromatin masses are splayed in fan-like orientations (arrowheads), and the antiparallel MTs are not engaged. (C) In a wild-type cytokinetic cell, phragmoplast MTs are arranged in parallel bundles. The mirror-imaged antiparallel bundles leave a very narrow dark line in the middle (arrows). (D) In a dyc283 phragmoplast, antiparallel MT bundles exhibit a fan-like pattern, and are not engaged with each other (arrowheads). Bar = 5 mm. at ;85 kD by immunoblotting against the wild-type extract (see Supplemental Figure 2 online). The dyc283 mutation wascaused by an insertion in the fifth exon of the gene encoding MAP65-3, and thus likely was null (Caillaud et al., 2008). Similar amounts of proteins extracted from the dyc283 seedlings, as indicated by the reference antiactin bands, were probed with the anti-MAP65-3 antibodies. No signal was detected at the position corresponding to the At MAP65-3 band, confirming that the dyc283 plant did not produce the protein (see Supplemental Figure 2 online). When the monospecific anti-MAP65-3 antibodies were used in immunofluorescent localization, mitotic cells after completing anaphase exhibited pronounced signals in the middle region of the central spindle and the phragmoplast midline (Figure 4). When the antibodies were applied to dyc283 cells, no noticeable signal was detected in the phragmoplast (see Supplemental Figure 3 online), indicating that they did not cross-react with other MAP65 isoforms. When cells progressed to the late stages of anaphase, interzonal MTs started to coalesce between segregating sister chromosomes (Figures 4A to 4C). Punctate anti– MAP65-3 signals were detected near the midzone MTs in the central spindle (Figure 4A). No noticeable anti–MAP65-3 signal was detected with kinetochore fibers containing parallel MTs. When kinetochore fibers were completely depolymerized, Figure 4. Localization of Endogenous MAP65-3 from Late Anaphase to Cytokinesis by Immunofluorescence. In merged images, MAP65-3 is pseudocolored in green, MTs in red, and DNA in blue. (A) to (C) At late anaphase, the midzone MTs are still not as prominent as the shortening kinetochore fibers. MAP65-3 is concentrated in the middle of the central spindle. (D) to (F) When sister chromosomes reach spindle poles, midzone MTs become highly bundled in parallel. MAP65-3 accumulated in the middle segments of the midzone MTs. Arrows indicate MAP65-3 association with the distal ends of MTs emanated from the spindle pole. (G) to (I) At telophase, when the antitubulin dark line (arrowheads) becomes visible in the middle of the developing phragmoplast, the localization of MAP65-3 becomes much more restricted to the midline of the phragmoplast. (J) to (L) At late cytokinesis, the phragmoplast becomes a ring surrounding the forming cell plate. MAP65-3 remains associated with the phragmoplast midline, where antiparallel MTs are present. Bar = 5 mm. Phragmoplast Microtubule Organization antitubulin line started to appear (arrowheads, Figure 4H). At this stage, MAP65-3 became restricted to the middle region of the central spindle and seemed to connect the two sets of bundled MTs (Figures 4G to 4I). Later, MTs radiating from spindle poles disappeared, and the central spindle was replaced by the phragmoplast. Throughout the development of the phragmoplast, MAP65-3 was always associated with the midline of the phragmoplast, wherever the MTs from the opposite sides of the division plane met (Figures 4J to 4L). Such a localization pattern persisted until the complete depolymerization of phragmoplast MTs. We analyzed the distribution patterns of MT bundles and MAP65-3 in the central spindle and the phragmoplast based on quantitative measurement of the fluorescent intensities. When the antitubulin intensity was measured along MT bundles in the central spindle, it was found that the signal dropped toward the middle segment of such MT bundles (Figures 5A and 5B). The reduction in antitubulin signal was coincident with the rise of antiMAP65-3 signal (Figures 5A and 5B). The reversed changes of antitubulin and anti-MAP65-3 signals remained when MAP65-3 became restricted to a narrow region in the central spindle (Figures 5C and 5D). At this stage, as well as during cytokinesis, the antitubulin fluorescence away from the midline became striking. In comparison, the signal in the midline was so low that it was almost invisible in images exposed according to the fluorescent intensity in the rest of the phragmoplast. Our measurement showed that the intensity of the background fluorescence was less than half of that of the lowest point of antitubulin fluorescence. Thus, MTs did cross the midline of the phragmoplast. MAP65-3 Alone Is Sufficient to Cross-Link MTs In Vitro It was suggested in the CPAM model that MAP65 could be among the CPAM-associated proteins that interact with MT plus ends (Austin et al., 2005). If MAP65-3 were associated with CPAM, we would expect it to form an end-on interaction with MT plus ends. To test this possibility, a bacterially expressed 63HisGFP-33FLAG-MAP65-3 fusion protein was prepared, and the full-length fusion protein and a degradation product were purified by successive affinity chromatography and gel filtration (Figure 6A). The tendency of this MAP65 family of proteins to dimerize might be the reason that the full-length and truncated proteins copurified with each other. When they were tested for MT binding, both the intact (arrow, Figure 6B) and the degradation product (arrowhead) exhibited strong binding to bovine brain MTs as demonstrated by the MT cosedimentation experiment (Figure 6B). In the absence of MTs, at the maximum concentration of 0.3 mM MAP65-3, the fusion protein remained in the supernatant. To test whether MT cosedimentation of the fusion protein was accompanied by MT bundling, 2.7 mM fluorescently labeled MTs were observed inthe presence of 0.0125–0.1 mM 63His-GFP-33FLAG-MAP65-3. As a negative control, 0.1 mM glutathione S-transferase (GST)-GFP-33FLAG was incubated with MTs, and this did not cause any obvious MT bundling effect (Figure 6C). MTs were seen as discrete single filaments in cases of MTs alone or plus GST-GFP-33FLAG (Figures 6C and 6D). In the presence of 63His-GFP-33FLAG-MAP65-3, MT bundles appeared (Figure 6C). Concomitant with the greater amount 2913 Figure 5. Analysis of Antiparallel MT Bundles and Associated MAP65-3. Merged images have MTs in red and MAP65-3 in green ([A] and [C]). The regions subjected to intensity measurement are highlighted in white boxes. In the intensity plots ([B] and [D]), the x axis represents the distance in pixels along the boxed region, and the y axis represents intensity in gray levels. (A) and (B) In a late anaphase cell, the intensity of antitubulin immunofluorescence drops toward the middle of MT bundles, which coincides with the rise in MAP65-3 signal. Avg, average. (C) and (D) When MAP65-3 is restricted to the midline of the phragmoplast, the intensity of MTs drops sharply in the middle, but is still significantly higher than that of the background. Again, the drop in the antitubulin signal coincides with the rise in that of anti-MAP65-3. Bar = 5 mm. of 63His-GFP-33FLAG-MAP65-3, more MT bundles were observed. In the presence of 0.1 mM 63His-GFP-33FLAGMAP65-3, thick MT bundles were induced (Figure 6C). We also quantified bundles of MTs formed at different concentrations of 63His-GFP-33FLAG-MAP65-3. When the fusion protein was added at 0.0125 mM, ;11.6% MTs appeared in bundles (Figure 6D). Upon addition of 0.1 mM of 63His-GFP-33FLAG-MAP65-3, over 41.2% were in bundles (Figure 6D). We also tested whether MAP65-3 selectively bundled antiparallel MTs in vitro. Polarity-marked MTs were prepared before the addition of 63His-GFP-33FLAG-MAP65-3. All bundled MTs were decorated by the fusion protein. We selected bundled MTs that had new segments grown out of the MT seeds, so that the polarities of both MTs were clearly discerned. Among 24 bundled MT doublets, 21 had the polarities of both MTs clearly discerned, and all showed a clear antiparallel arrangement, as shown in Figure 7. The antiparallel MTs were bundled toward their plus ends, which faced inwardly (Figures 7C to 7F). The MT-overlapping regions were decorated by 63His-GFP-33FLAG-MAP65-3 (Figures 7A to 7D). In the meantime, the fusion protein was not detected along single and 2914 The Plant Cell Figure 6. Preparation of the 63His-GFP-33FLAG-MAP65-3 Fusion Protein and MT Cosedimentation and Bundling Tests. (A) Purified 63His-GFP-33FLAG-MAP65-3 and GST-GFP-33FLAG proteins. The GFP-MAP65 lane has two major bands of full-length 63His-GFP33FLAG-MAP65-3 and a degradation product. The GFP lane has a single major band of GST-GFP-33FLAG. The molecular weight markers (M; in kD) are indicated on the left. (B) Cosedimentation of 63His-GFP-33FLAG-MAP65-3 with MTs. In the absence of MTs, the fusion protein appears in the supernatant. At 0.075–0.3 mM, both the full-length fusion protein (arrow) and the major degradation product (arrowhead) appear in the pellet fractions with MTs. The tubulin band is marked by the asterisk. The molecular weight markers (in kD) are indicated on the left. (C) MT bundling caused by the addition of 63His-GFP-33FLAG-MAP65-3. MTs by themselves appear in discrete single filaments. The addition of GSTGFP-33FLAG does not make a difference. When 63His-GFP-33FLAG-MAP65-3 is added, greater bundling can be seen when more fusion protein is added. (D) Quantification of MT bundling induced by 63His-GFP-33FLAG-MAP65-3. At 0.075 to 0.3 mM, more than 10 to 40% of MTs appear in bundles. free MTs (Figure 7D). These results suggest that MAP65-3 is sufficient to cross-link MTs in an antiparallel fashion in vitro, and consequently MT bundles can be induced. MAP65-3 Is Enriched near the Plus Ends of Antiparallel MTs Because MAP65-3 was detected at the bridging region of antiparallel MTs after late anaphase, we asked whether the MAP65-3–MT interaction only took place in the presence of antiparallel MTs in vivo. The temperature-sensitive Radially Swollen7 (rsw7) mutation at a locus encoding a Kinesin-5 motor causes bipolar spindles to collapse into monopolar ones at restrictive temperatures (Bannigan et al., 2007). In collapsed monopolar spindles in mutant cells, MAP65-3 was detected atthe distal plus ends of MTs radiating away from the single pole (arrowheads, Figure 8). Occasionally, fragments of MTs attached to monopolar spindles exhibited a bipolar appearance, as indicated by a dark line by antitubulin immunofluorescence (arrow, Figure 8). In such fragments, the minus ends of the antiparallel MTs were located toward the distal ends when revealed by antig-tubulin staining (see Supplemental Figure 4 online). A more pronounced MAP65-3 signal was detected in association with MTs in the middle region of the antiparallel MTs when compared with the signal associated with distal ends of emanating MTs (Figure 8). Therefore, the result indicates that MAP65-3 specifically recognizes MT segments near the plus end at later stages of mitosis, and the association becomes greatly enhanced when encountering the plus ends of antiparallel MTs. Accumulation of MT Plus Ends in the Phragmoplast Midline Is Dependent on MAP65-3 Because the loss of MAP65-3 leads to the widening of the dark line that is visualized by antitubulin immunofluorescence (Müller et al., 2004), we asked whether the phenotype was caused by defects in MT plus end distribution. The functional Phragmoplast Microtubule Organization 2915 line while also present elsewhere in both mature and a late phragmoplasts (Figures 9A to 9F). In the dyc283 mutant cells, however, EB1c-33FLAG-GFP no longer accumulated in the widened midlines in phragmoplasts of similar stages (Figures 9G to 9L). Instead, the signal became more or less evenly distributed in the phragmoplast region and elsewhere in the cell. Hence, we conclude that MAP65-3 is required for the rich presence of MT plus ends in the middle region of the phragmoplast. MAP65-3 Is Required for MT Plus End Localization of Kinesin-12 and PAKRP2 in the Phragmoplast Our previous work showed that the MT motor Kinesin-12 exhibits exclusive localization at MT plus ends in the phragmoplast (Lee and Liu, 2000; Pan et al., 2004; Lee et al., 2007). We asked whether it colocalized with MAP65-3. A transgenic line was established to express a MAP65-3-FLAG fusion protein under its native promoter. The seedling growth retardation and Figure 7. MAP65-3 Preferentially Bundles Antiparallel MTs In Vitro. Two examples are shown. (A) and (D) Fluorescent images of MT seeds MTs (first row), Rhodamine X–labeled MTs (second row), MAP65-3 (third row), and merged images (fourth row) with MT seeds in blue, newly grown out MTs in red, and 63His-GFP-33FLAG-MAP65-3 in green. (B) and (E) Fluorescence intensity scans across the areas of bundled MTs in (A) and (D) with the starting point marked by an arrow and the ending point by an arrowhead, respectively. The x axis represents distance in mm, and the y axis represents gray level in an 8-bit scale. (C) and (F) The diagrams depict the positions of MT seeds, elongated segments of MTs, and At MAP65-3. The keys for the diagrams are shown on the bottom. Bar = 5 mm. EB1c-33FLAG-GFP was expressed in the dyc283 mutant background, and its localization was compared with that of control cells expressing the identical fusion protein. In control cells, similarly to what has been shown in live cells, the EB1c-33FLAGGFP signal was particularly abundant in the phragmoplast mid- Figure 8. Antiparallel MT Bundles Enrich MAP65-3 In Vivo. A monopolar spindle was generated in the rsw7 mutant cell at the restrictive temperature. The merged image has MTs in red, MAP65-3 in green, and DNA in blue. MAP65-3 can be seen in the distal ends of radiating MTs of an aster-like array (arrowheads). Occasionally, MTs are arranged in an antiparallel fashion, and the fortuitous phragmoplast-like MTs have their middle line (arrows) decorated by MAP65-3 with greater signal strength than the protein appeared at the MT distal ends of the asters. Bar = 5 mm. 2916 The Plant Cell (arrows, Figure 10F). However, wherever Kinesin-12A was detected, MAP65-3 was also present. The loss of engagement of the plus ends of antiparallel MTs in the dyc283 phragmoplast prompted us to examine whether the localization of phragmoplast-specific kinesins was affected in the mutant cells. Compared with control cells, in which Kinesin-12A exclusively decorated the phragmoplast midline (Figure 11A, a to c; 100%, n = 16), it was no longer concentrated in the phragmoplast of the dyc283 mutant cells. Instead, the anti– Kinesin-12A signal became largely dispersed in the cytoplasm (Figure 11A, d to f; 79%, n = 115). Phragmoplast-Associated Kinesin-Related Protein2 (PAKRP2) has a wider localization pattern in the phragmoplast than Kinesin-12 (Figure 11B, a to c.) (Lee et al., 2001). Nevertheless, its signal was more pronounced toward the phragmoplast midline than elsewhere in the phragmoplast (Figure 11B, a to c; 100%, n = 55). In the dyc283 mutant cells, however, PAKRP2 was no longer concentrated in the phragmoplast midline (Figure 11B, d to f). Instead, it evenly decorated phragmoplast MTs in a punctate manner, leaving behind a dark line in the middle (Figure 11B, d to f; 94%, n = 72). Thus, these results suggest that MAP65-3 is required for the interaction of these kinesin motors with MT plus ends in Arabidopsis. MAP65-3 Is Not Essential for Cell Plate–Destined Membrane Trafficking Various membrane trafficking markers have been shown to specifically decorate the phragmoplast midline (Bednarek and Figure 9. Localization of EB1c-33FLAG-GFP in Control and dyc283 Cells by Immunofluorescence. The merged images have EB1c-33FLAG-GFP in green, MTs in red, and DNA in blue. (A) to (F) In a control cell containing a mature phragmoplast ([A] to [C]) and another one at late cytokinesis ([D] to [F]), EB1c-33FLAG-GFP is abundantly present along antiparallel MTs. Its signal is particularly pronounced in the phragmoplast midline, which is shown in dark by tubulin immunofluorescence. (G) to (L) In dyc283 cells at similar stages as the control cells, the EB1c33FLAG-GFP signal no longer accumulated in the phragmoplast midline. Bar = 5 mm. cytokinetic defects caused by the dyc283 mutation were completely suppressed upon the expression of this fusion (see Supplemental Figure 5 online). This line allowed us to detect both MAP65-3 and Kinesin-12A in the same cells (Figure 10). In a late anaphase cell, MAP65-3 was found to decorate underlying MT bundles in the central spindle; however, the Kinesin12A signal was barely detectable at the same location, but largely diffused in the cytoplasm (Figures 10A to 10C). This result suggests that MAP65-3 likely precedes Kinesin-12A to interact with midzone MTs in the central spindle. In a cell undergoing cytokinesis, both proteins exhibited exclusive decoration of the midline of the phragmoplast (Figures 10D and 10E). However, the two proteins did not completely overlap as visualized in the merged image, in which there were areas where only MAP65-3 was seen Figure 10. Dual Localization of MAP65-3 and Kinesin-12A by Immunofluorescence with Anti-FLAG for the MAP65-3-FLAG Fusion Protein and Anti-Kinesin-12. The merged images have Kinesin-12A in green, MAP65-3 in red, and DNA in blue. (A) to (C) In a late anaphase/telophase cell, MAP65-3 is already associated with bundled MTs, but Kinesin-12A is barely visible. (D) to (F) In the phragmoplast, both signals are concentrated in the midline. However, the MAP65-3 signal does not completely overlap with that of Kinesin-12A. Kinesin-12A is absent from some of the regions containing MAP65-3 signal (arrows). Bars = 5 mm. Phragmoplast Microtubule Organization 2917 et al., 1997). In the dyc283 mutant cells undergoing cytokinesis, KNOLLE again predominantly appeared in the cell division site, and the signal often appeared in thicker lines than in the control cells (Figures 12D to 12F; 100%, n = 37). Similarly, the dynamin Dynamin-Related Protein 1A (DRP1A) was found to be concentrated in the phragmoplast midline in the dyc283 cells as in the control cells (see Supplemental Figure 6 online). Thus, these results indicate that the membrane trafficking required for cell plate formation is not significantly affected by the absence of MAP65-3, even though the MT plus ends become disengaged under such conditions. DISCUSSION Our results show that in the phragmoplast, antiparallel MTs often have their plus ends engaged with each other, and active MT polymerization takes place at the cell division site. Our results also suggest that MAP65-3 functions in cross-linking antiparallel MTs near their plus ends during telophase and cytokinesis. Direct evidence was provided here indicating that only overlapping MTs interact with MAP65-3. Although other MAP65 isoforms have been implicated in cross-linking antiparallel MTs in the phragmoplast (Van Damme et al., 2004b; Smertenko et al., 2008), MAP65-3 apparently plays a more critical role than other isoforms. The MT cross-linking activity of MAP65-3 is required for the interaction of kinesins that are important for cytokinesis with MT plus ends, and is consequently critical for cytokinesis. MT Organization in the Central Spindle and the Phragmoplast In the central spindle at late anaphase and telophase, long segments of antiparallel MTs overlap toward the midzone and Figure 11. Requirement of MAP65-3 for the Localization of Kinesin-12 and PAKRP2 at MT Plus Ends in the Phragmoplast. (A) Localization of Kinesin-12A in the wild-type and dyc283 mutant cells. The merged images have Kinesin-12A in green, MTs in red, and DNA in blue. Kinesin-12A exclusively decorates the midline of the phragmoplast MTs in a wild-type control cell ([a] to [c]). Kinesin-12A is no longer detected in the phragmoplast in dyc283 cells ([d] to [f]). (B) Localization of PAKRP2 in the wild-type and dyc283 mutant cells. The merged images have PAKRP2 in green, MTs in red, and DNA in blue. PAKRP2 is associated with phragmoplast MTs with greater emphasis near the midline of the phragmoplast in a wild-type control cell ([a] to [c]). Puctate PAKRP2 signal can be seen among phragmoplast MTs in a dyc283 cell ([d] to [f]). The PAKRP2 signal appears along phragmoplast MTs, and does not accumulate at the division site in a dyc283 cell ([g] to [i]). Bars = 5 mm. Falbel, 2002; Jürgens, 2005). We wondered whether the changes in MT organization caused by the loss of MAP65-3 would alter membrane trafficking patterns. The membrane fusion SNARE protein KNOLLE appeared at the phragmoplast midline as shown before (Figures 12A to 12C; 100%, n = 27) (Lauber Figure 12. Accumulation of the Syntaxin KNOLLE in the Phragmoplast Midline in the Absence of MAP65-3. Triple labeling of KNOLLE ([A] and [D]), MTs ([B] and [E]), and DNA in cytokinetic cells of the wild-type control ([A] to [C]) and dyc283 mutant ([D] to [F]). The merged images ([C] and [F]) have KNOLLE pseudocolored in green, MTs in red, and DNA in blue. KNOLLE can be detected in the midline (arrows) of phragmoplast MTs in both the wild-type (A) and dyc283 mutant (D) cells. Bar = 5 mm. 2918 The Plant Cell are cross-linked by factors such as MAP65-3. Through an unknown mechanism, the overlapping region of these interdigitating MTs (IMTs) is gradually reduced while the cell progresses toward cytokinesis. Although MAP65-3 becomes restricted to a narrow region when a typical phragmoplast MT array is established, the MAP65-3 signal is believed to reflect the presence of a narrow MT-overlapping region. The decrease in MT density seems to be coincident with the localization of MAP65-3, which highlights the cross-linked antiparallel MTs. In the Haemanthus endosperm cells as well as the moss protenema cells, antiparallel MTs clearly interdigitate or overlap near their plus ends in the phragmoplast (Hepler and Jackson, 1968; Hiwatashi et al., 2008). In root meristematic cells in Arabidopsis, however, electron tomographic results showed that antiparallel MTs were largely separated by membranous sacks of CPAM enclosing large quantities of carbohydrates (Austin et al., 2005). This discrepancy had left us with the question of whether MT organization patterns are different in phragmoplasts of different cells. Based on our biochemical, TEM, and fluorescent imaging data, we provide direct evidence that a subpopulation of antiparallel MTs indeed interdigitates in the phragmoplast. The interdigitation is brought about by the MT– cross-linking factor MAP65-3. Our data do not contradict the finding that the majority of antiparallel MTs do not interdigitate in the phragmoplast. How do we explain the appearance of a dark line in the middle of the phragmoplast in both antitubulin immunofluorescence and GFP-tubulin images? The dark line is likely caused by an imaging artifact due to the sharp difference in fluorescence intensity between the midline and the rest of the phragmoplast. The linescan of fluorescent intensity showed that the MT signal intensity in the midline was significantly higher than the background signal. However, when the neighboring fluorescent signal was overwhelmingly strong because of the abundance of MTs, the midline seemed dark due to significantly fewer MTs. A similar example can be found in the study of the Kinesin-14 member Arabidopsis thaliana Kinesin 5 (ATK5) in prometaphase spindles (Ambrose and Cyr, 2007). Upon the breakdown of the nuclear envelope, MTs, highlighted by an MBD (MT binding domain)-DsRed, were organized into a spindle configuration with the DsRed signal largely seen at the spindle periphery and spindle pole region. However, the yellow fluorescent protein (YFP)-ATK5 signal occupied the middle region of the spindle, as the fusion protein decorated MT bundles. Although YFP-ATK5 seemed to be present in areas devoid of MTs, the linear appearance of the signal suggests that this labeling was most likely due to the presence of underlying MTs in the area. Hence, we conclude that MTs are present in the region of the phragmoplast midline. A Modular Model of the Phragmoplast MT Array Based on the findings presented here, we postulate a model depicting the organization of antiparallel MTs in both the central spindle and the phragmoplast (Figure 13). In this modular model, both IMTs and noninterdigitating MTs (nIMTs) are represented, but nIMTs outnumber IMTs. IMTs and nIMTs are integrated together to form modules. Each module has the IMTs in the center surrounded by nIMTs. Earlier modules are established during telophase, when the central spindle contains conspicuous MT bundles. These MT bundles have lower MT density toward the middle. Such a modular model echoes the mini-phragmoplast concept that was introduced when describing clusters of antiparallel MTs during endosperm cellularization (Otegui and Staehelin, 2000). We would like to refer to each modular cluster of IMTs and nIMTs as a mini-phragmoplast MT array (Figure 13). In this array, MAP65-3 as well as Kinesin-12 only associate with IMTs toward their plus ends. Because of the cross-linking effect, IMTs are predicted to be highly stable, and their plus ends remain at the phragmoplast midline. Among the MAP65 members that decorate phragmoplast MTs, MAP65-3 has the most restricted association with the phragmoplast midline (Van Damme et al., 2004a; Van Damme et al., 2004b). Compared with other MAP65 members, MAP65-3 exhibits the slowest turnover rate (Smertenko et al., 2008), suggesting stable association with IMTs. Both IMTs and nIMTs undergo robust MT polymerization, as demonstrated by the EB1c activity. During cytokinesis, the phragmoplast MT array expands centrifugally toward the cell periphery. The older parts of the array, toward the center, gradually disappear, while the new ones appear as if the MTs of the new modules assemble at the same rate as the MTs of the old modules disassemble. How do the mini-phragmoplast modules propagate? This is believed to be accomplished by the robust polymerization of nIMTs and their interactions with cross-linkers, like MAP65-3. ATK5 may be one of the factors that promotes rapid MT polymerization at the advancing edge of the phragmoplast, as suggested by its preferential localization to this region (Ambrose et al., 2005). Once MAP65-3 binds to the plus-end regions of these MTs, it would seek to interact with antiparallel MTs initiated from the opposite side of the phragmoplast. One may also consider that newly polymerized antiparallel MTs could be captured by MAP65-3 already associated with extant ones. Based on our in vitro results, we hypothesize that, once bound to antiparallel MTs, MAP65-3 undergoes self-oligomerization by recruiting more of its dimers. The antiparallel MTs cross-linked by MAP65-3 are then stabilized and become IMTs. Concomitantly, nIMTs continue to undergo rapid polymerization and depolymerization. It is worth noting that in Haemanthus endosperm cells, accessory phragmoplasts can be formed when the cells are incubated at elevated temperatures (Bajer et al., 1993). Pronounced MT elongation took place under these conditions. One could interpret the formation of accessory phragmoplasts as being due to the establishment of an antiparallel array resulting from fortuitous interactions between MTs that will be stabilized upon interactions with proteins like MAP65-3. During cellularization in the endosperm, the mini-phragmoplast contains clusters that each consist of ;10 overlapping MTs (Otegui and Staehelin, 2000). Surprisingly, nonoverlapping MTs were not obvious in this case. Such a feature may be unique to the formation of a syncytial-type cell plate. In somatic cells, however, the nIMTs become dominant. Whether this difference in MT organization between endosperm cellularization and somatic cytokinesis reflects the difference in cell plate formation remains to be tested. Phragmoplast Microtubule Organization 2919 Figure 13. A Model Depicting MT Organization in the Central Spindle at Late Anaphase or Early Telophase and the Phragmoplast during Cytokinesis. The keys of the diagrams are included in the box. Two populations of MTs, IMTs and nIMTs, are present at both stages. MAP65-3 and Kinesin-12 only associate with IMTs near their plus ends. EB1 decorates polymerizing MT plus ends of both IMTs and nIMTs. Plus (+) and minus (2) ends of MTs are illustrated. Functions of Other MAP65 Family Proteins in the Phragmoplast Although MAP65-3 plays a critical role in phragmoplast MT organization, other MAP65 members also are detected in the Arabidopsis phragmoplast by GFP tagging and immunofluorescence (Smertenko et al., 2000; Van Damme et al., 2004a). However, their localization patterns differ from each other. Unlike MAP65-3, for example, a MAP65-1-GFP fusion protein did not appear in the midzone but along phragmoplast MTs elsewhere (Gaillard et al., 2008). Because of their common cross-linking and bundling activity (Smertenko et al., 2004; Mao et al., 2005; Gaillard et al., 2008; Smertenko et al., 2008), it is intriguing to determine whether MAP65 isoforms exhibit functional redundancy in the phragmoplast. However, mutations in genes encoding MAP65-1 and -2 only cause obvious phenotypes in elongating cells (Lucas et al., 2011). In tobacco, the MAP65-1 protein, which is highly similar to the Arabidopsis MAP65-1, plays a critical role in MT turnover in the phragmoplast (Sasabe et al., 2006). Its phosphorylation by a MAP kinase downregulates its MT-bundling activity and consequently promotes the destabilization of MTs and expansion of the phragmoplast. In A. thaliana, MAP65-1, -2, and -3 all have been shown to serve as putative substrates of a similar kinase at the phragmoplast midzone (Sasabe et al., 2011). Moreover, mutations in the genes encoding MAP65-1 or -2 would enhance the cytokinetic phenotype caused by the loss of MAP65-3, suggesting that there is functional redundancy among the three homologous proteins (Pesquet and Lloyd, 2011). However, it is unclear how phragmoplast MTs are altered in these double mutants when compared with single mutants like dyc283. It would be interesting to test whether ectopic expression of MAP65-1 or -2 could suppress the cytokinetic phenotypes in dyc283 cells. Maintenance of the Bipolar Configuration of Phragmoplast MTs The localization of MAP65-3 could imply that it might be critical for maintaining the bipolar arrangement of antiparallel MTs in the phragmoplast. However, genetic data indicate that MAP65-3 is not required for either establishing or maintaining the bipolar pattern. Its absence causes antiparallel MTs to be disengaged with each other, as demonstrated by a wide gap in the phragmoplast midline in the ple mutant (Müller et al., 2004). The loss of such close engagement seriously compromises the effectiveness of cell plate formation, but does not completely abolish the process. How do highly dynamic MTs remain in a bipolar configuration in the phragmoplast? The bipolarity may be brought about by the unidirectional MT polymerization toward the division site in the phragmoplast. It still remains to be determined how the phragmoplast prevents MTs from being polymerized toward the opposite direction. When exogenous tubulins were supplied to permeabilized cells containing phragmoplasts, the added tubulin subunits predominantly localized to the phragmoplast midline, presumably onto the plus ends of IMTs (Vantard et al., 1990; Asada et al., 1991). These observations seem to be contradictory to the scenario of robust polymerization taking place for both IMTs and nIMTs. We assumed that the result was due to the loss of nIMTs caused by harsh experimental conditions, because both experiments required a permeabilization step to allow tubulins 2920 The Plant Cell to freely enter the cells. IMTs likely exhibit higher stability than nIMTs, because of being stabilized by MAP65-3. When more tubulin dimers were added to the plus ends of IMTs, these MTs tended to grow across the phragmoplast midline. If the plus ends were not properly positioned, cytokinesis failed due to irregular deposition of cell plate materials (Lee et al., 2007). The function of the Kinesin-12 motor is to keep the plus ends positioned at the division site. Although the initial sliding of central spindle MTs depends on Kinesin-5, additional segments added to IMTs are pushed apart by Kinesin-12 (Lee et al., 2007). Other MT-interacting factors, like EB1 and Microtubule Organization1, may not directly contribute to the establishment of the bipolar phragmoplast MT array (Eleftheriou et al., 2005; Kawamura et al., 2006). However, they contribute to other processes, like the regulation of polymerization and depolymerization, as well as the stability of the MTs. These and other MT-interacting factors become localized in the phragmoplast when they interact with MTs there. Before interacting with MTs, they would diffuse in the cytoplasm. Interaction of Proteins at MT Plus Ends in the Phragmoplast Our results show that stable interactions with MT plus ends in the phragmoplast require the IMTs and nIMTs probably undergo rapid turnover. Motor proteins, like Kinesin-12 and PAKRP2, exhibit conspicuous localization to the MT plus ends in the phragmoplast (Lee and Liu, 2000; Lee et al., 2001; Pan et al., 2004). When IMTs were lost, their plus end association was compromised. If the motor accumulated at the plus end by active movement, it would become evenly distributed along MTs when the IMTs were lost, as seen in PAKRP2 here. However, if a motor was directly bound to the plus ends without walking along MTs, its localization would be completely lost in the absence of IMTs, as seen in Kinesin-12. It is unknown whether MAP65-3 directly interacts with Kinesin-12 at MT plus ends. In summary, our results suggest that MAP65-3 decorates IMTs but not nIMTs in the phragmoplast. Successful cytokinesis requires the activity of both IMTs and nIMTs. METHODS Plant Materials, Growth Conditions, and Transformation Arabidopsis thaliana materials used in this study include the wild-type (Columbia-0) plants, and the dyc283 (Caillaud et al., 2008), rsw7 (Bannigan et al., 2007), and eb1c (Bisgrove et al., 2008) mutants. Plant growth conditions and agrobacterium-mediated transformation are as described previously (Kong et al., 2010). For observation of the EB1c-33FLAG-GFP and MAP65-3-FLAG fusions, seeds were germinated on 0.8% phytagel (Sigma-Aldrich) agar plates containing half-strength Murashige and Skoog medium (SigmaAldrich) with 0.5 g/L MES (Acros) (pH 5.8) after stratification in the dark at 48C. Oryzalin (Sigma-Aldrich) was added to the germination medium at 100 nM to test the hypersensitivity of the eb1c mutant to the drug (Bisgrove et al., 2008). Establishment of Lines Expressing MAP65-3-FLAG and EB1c-33FLAG-GFP A MAP65-3-FLAG fusion protein was expressed under the native At MAP65-3 promoter. To do so, the 1.3-kb promoter region was amplified by PCR using the Phusion High-Fidelity DNA polymerase (New England Biolabs) and primers AtMAP65 1: 59-CAC CAC ACT CTT CCC TAC ACA AAA CCG C-39 and AtMAP65 2: 59-GAA GAA TCG GAT CTT TTT GAA CAC TTG CCA T-39. The MAP65-3-coding sequence was amplified using the full-length cDNA clone RAFL09-53-C05 (Seki et al., 1998; Seki et al., 2002) as the template and the primers AtMAP65 3: 59-ATG GCA AGT GTT CAA AAA GAT CCG ATT CTT C-39 and AtMAP65 8: 59-AAC CAA ACG ACA TTC AGA CTG TAG CAT G-39. The resulting two DNA fragments were fused by an additional PCR reaction using the primers AtMAP65 1 and AtMAP65 8. The fused product was cloned into the Gateway pENTR/D-TOPO vector (Invitrogen), followed by cloning into the destination vector pGWB10 (Nakagawa et al., 2007) by an LR recombination reaction according to the manufacturer’s instructions, rendering a FLAG tag in the C terminus after expression. The resulting plasmid was transformed into the dyc283 mutant to test for genetic suppression/ complementation. To detect EB1c in live cells, an EB1c-33FLAG-GFP fusion protein was expressed under the control of its native promoter. Briefly, a 2399-bp genomic fragment encompassing a 665-bp promoter region plus the EB1c-coding sequence was amplified by PCR with the Phusion polymerase using the primers EB1c-F2: 59-CAC CGC GGC CGC TTG GAC AGG TTA ATG GGC TTG TG-39 and EB1c-R2: 59-GTC ATC TAG AGC AGG TCA AGA GAG GAG ATG AAC C-39 (the NotI and XbaI sites are underlined). The PCR product was cloned into the p33FLAG-CMV-14 vector (Sigma-Aldrich) at the NotI and XbaI sites. The At EB1c genomic sequence together with the 33FLAG-coding sequence was subsequently amplified together by the primers EB1c-F2 and FLAG33-R1: 59-CTT GTC ATC GTC TCC TTG TAG TC-39. The resulting fragment was cloned into pENTR/D-TOPO and then into the pGWB4 vector (Nakagawa et al., 2007) by an LR recombination reaction. The resulting plasmid was transformed into eb1c mutant plants. Transformants were tested for genetic suppression against hypersensitivity to 100 nM oryzalin. The same plasmid was transformed into the dyc283 mutant plants. Fusion Protein and Antibody Production To produce a GST-MAP65-3 fusion protein as an antigen, the HindIII fragment of the cDNA encoding amino acid 161-487 was cleaved from the RAFL09-53-C05 plasmid and cloned into the pGEX-KG vector (Guan and Dixon, 1991) at the HindIII site. The GST-MAP65-3161-487 fusion protein was expressed in BL21 (DE3) pLysS cells (EMD Biosciences Novagen) and purified using immobilized glutathione resin (Pierce Chemical Co.) before being used for antibody production at the Comparative Pathology Laboratory on the University of California-Davis campus. Specific antibodies against MAP65-3 were purified using columns of GST and GST-MAP653161-487 proteins, which had been immobilized on agarose using the AminoLink Plus Kit (Pierce Chemical Co.), according to the manufacturer’s instructions. Briefly, anti-GST antibodies were depleted from the antiserum by the GST column. The flow-through was applied to the GSTMAP65-3161-487 column, and subsequently specific antibodies were eluted from the column with 100 mM glycine (pH 2.5). Purified antibodies were immediately neutralized with 1/10 volume of 1 M Tris HCl (pH 8.0). To produce a full-length MAP65-3 fusion protein, the MAP65-3–coding sequence was amplified from the cDNA clone RAFL09-53-C05 with the primers At MAP65-3 5XbaI: 59-CAC CTC TAG AGC AAG TGT TCA AAA AGA TCC GAT TC-39 and AtMAP65-3 3BHI: 59-GAT CGG ATC CTC AAA CCA AAC GAC ATT CAG ACT GT-39 (the XbaI and BamHI sites are underlined). The PCR product was cloned into the p33FLAG-CMV-7 vector (Sigma-Aldrich) at the XbaI and BamHI sites. The 33FLAGMAP65-3 fragment was then amplified by the primers N-33FLAG-f1: 59-CAC CGA CTA CAA AGA CCA TGA CGG TGA TTA TAA-39and AtMAP65-3 3BHI. The resulting fragment was cloned into pENTR/DTOPO, and subsequently into the pGWB6 vector (Nakagawa et al., 2007) by an LR recombination reaction. The coding sequence of the Phragmoplast Microtubule Organization GFP-33FLAG-MAP65-3 fusion was amplified by PCR using primers GFP5KpnI: 59-CAC CGG TAC CAT GGT GAG CAA GGG CGA GGA GCT G-39 and AtMAP65-3 3GXhoI: 59-CAT CTC GAG CAA CCA AAC GAC ATT CAG ACT GTA GCA TG-39. After digestion with KpnI and XhoI, the fragment was cloned into the pET30a vector at the corresponding sites. The ligation product was then transformed into Rosetta2 competent cells (Novagen). The expressed 63His-GFP-FLAG-MAP65-3 fusion protein was purified using Ni Sepharose 6 Fast Flow according to the manufacturer’s instructions (GE Healthcare Life Sciences). The protein was further purified by gel filtration using HiPrep Sephacryl S-300 (GE Healthcare Life Sciences) according to a published protocol (Tao and Scholey, 2010). To prepare a protein to be used as a negative control in the in vitro MT binding assay, a GST-GFP-33FLAG fusion construct was made by digesting the GST-GFP-33FLAG-MAP65-3 fusion construct with BamHI and XbaI. The 0.7-kb fragment containing the GFP-33FLAG-coding sequence was cloned into the pGEX-KG vector at the BamHI and XbaI sites. Expressed fusion protein was purified as described above. 2921 in liquid nitrogen with a mortar and pestle. A trichloroacetic acid precipitation method was used to extract proteins as described previously (Lee et al., 2001). Extracted proteins were separated on a 7.5% SDS-PAGE gel and processed for immunoblotting analysis. Purified anti-MAP65-3 antibodies and the 3H11 antiactin monoclonal antibody (Andersland et al., 1994) were used to probe the blots. The antibodies were diluted at 1:1000 in Hikari reagent (Nacalai Tesque). Secondary antibodies were HRP-conjugated anti-rabbit and anti-mouse antibodies (Bio-Rad). Signals were detected by the chemiluminescent method of exposing an X-ray film on the blots. TEM Examination of the Dividing Cells of Roots Seedlings grown on the agar plate were rapidly frozen using an HPM 010 high-pressure freezing machine (Bal-Tec Products). Frozen samples were freeze-substituted according to a published protocol (van de Meene et al., 2006). Roots were embedded in Spurr’s resin (Ted Pella) and sectioned using a microtome (Leica). After staining with uranium acetate and lead citrate, root sections were observed under a 410 LS TEM (Philips). MT Cosedimentation and In Vitro MT Binding Assays Purified 63His-GFP-33FLAG-MAP65-3 and GST-GFP-33FLAG proteins were dialyzed against BRB80 (80 mM Pipes, 1 mM MgCl2, 1 mM EGTA, pH 6.8) and centrifuged at 100,000 3 g for 15 min at 48C to remove aggregations before being used for MT cosedimentation and in vitro MT binding assays. A MT cosedimentation assay was performed as described previously (Lee and Liu, 2000). The binding reaction was performed in a 38-mL reaction volume containing 2.7 mM MTs and 0.075, 0.15, or 0.3 mM 63HisGFP-33FLAG-MAP65-3 in the presence of 10 mM taxol in BRB80. As negative control experiments, the same assay was performed in the absence of either MTs or 63His-GFP-33FLAG-MAP65-3. For in vitro MT binding assays, fluorescent MTs were polymerized from Rhodamine X–labeled bovine brain tubulin (Cytoskeleton) and stabilized in 20 mM taxol in BRB80 supplemented with 1 mM GTP and 10% glycerol. The binding reaction was performed in a 20-mL reaction volume containing 0.0125, 0.025, 0.05, or 0.1 mM 63His-GFP-33FLAG-MAP65-3 with MTs polymerized from 0.06 mM Rhodamine X–labeled bovine brain tubulin (Cytoskeleton) in the same buffer. The mixture was immediately loaded onto a glass slide and observed under an Axiovert 200 inverted fluorescent microscope with a 403 Plan-Apo objective (Carl Zeiss). All the images were taken within 10 min of 63His-GFP-33FLAG-MAP65-3 being mixed with MTs. As a negative control, 0.0125, 0.025, 0.05, or 0.1 mM of the GST-GFP-33FLAG fusion protein was incubated with an identical amount of MTs as above. The number of bundled MTs was counted by analyzing the fluorescent intensity of the Rhodamine X in the filaments in each image. Bundled MTs were defined as filaments whose fluorescence intensity was at least twice as high as that of a single MT. The MT bundling index was defined as the number of bundled MTs divided by the number of all the MT filaments in each image. For each mixture, the bundling index was calculated with at least 200–300 filaments. The average of three independent tests was plotted. For the in vitro MT binding/bundling assay using polarity-marked MTs, polarity-marked MTs were prepared by incubating Rhodamine X–labeled bovine brain tubulin with short MT seeds polymerized from HiLyte 647labeled porcine brain tubulin (Cytoskeleton) for 1 h at 378C and stabilized in 20 mM taxol in BRB80 supplemented with 1 mM GTP and 10% glycerol. The binding/bundling reaction was performed in a 20-mL reaction volume containing 0.025 mM His-GFP3FLAG-MAP65-3, and polarity-marked MTs were polymerized from 0.06 mM tubulin in the same buffer. Observations were performed as described above. Fluorescent and Confocal Microscopy Root tip cells were processed for indirect immunofluorescence staining as described previously (Lee and Liu, 2000). Besides anti-MAP65-3 antibodies, other primary antibodies include DM1A anti–a-tubulin (SigmaAldrich), sheep antitubulin (Cytoskeleton), anti–PAKRP1-C for Kinesin-12 (Lee and Liu, 2000), anti-PAKRP2 (Lee et al., 2001), anti-KNOLLE (Rose Biotechnology), anti-FLAG (Shanghai Genomics), and anti-DRP1A (Kang et al., 2001). Secondary antibodies were Texas Red-conjugated donkey anti-mouse IgG (Molecular Probes) and FITC-conjugated donkey antirabbit IgG (Rockland Immunochemicals). Slides were observed under an Eclipse E600 microscope equipped with epifluorescence optics (Nikon) and ET filter sets (Chroma Technology Corp.). Images were captured by an Orca CCD camera (Hamamatsu Photonics) driven by the MetaMorph software package (Molecular Devices) before being assembled by the Photoshop software (Adobe). Z-stacks were taken under a DeltaVision Real Time Deconvolution Microscope (Applied Precision), and images were processed using the associated API softWoRx Explorer Suite. The EB1c-33FLAG-GFP signal was visualized in the root tip cells of 3-to 4-day-old seedlings with their roots mounted in 0.3 M mannitol. Time-lapsed images were taken using an FV1000 confocal laser scanning microscope (Olympus USA) with a PLAPON 603 oil objective lens and using the 488-nm argon laser for GFP excitation. The GFP signal was collected at the 500–550 nm range. Acquired images were processed using MetaMorph (Molecular Devices) and Photoshop (Adobe). Accession Numbers The Arabidopsis Information Resource (TAIR) locus identifier for the genes mentioned in this study are At5g51600 encoding At MAP65-3 and At5g67270 for At EB1c. The cDNA clone RAFL09-53-C05 contains the full-length coding sequence of At MAP65-3. Supplemental Data The following materials are available in the online version of this article. Supplemental Figure 1. Genetic Suppression/Complementation of the Eb1c Mutation by Expressing an EB1c-33FLAG-GFP Fusion Protein. Immunoblotting Experiments Supplemental Figure 2. Detection of the Endogenous MAP65-3 Protein by Immunoblotting. To extract proteins for an immunoblotting assay, developing flower buds were collected from the wild-type and dyc283 plants and frozen and ground Supplemental Figure 3. Negative Control for the Anti-MAP65-3 Immunofluorescence. 2922 The Plant Cell Supplemental Figure 4. Antiparallel MT Bundles in an rsw7 Mutant Cell. Supplemental Figure 5. Suppression of the dyc283 Mutation by Expressing MAP65-3-FLAG. Supplemental Figure 6. Localization of the Dynamin DRP1A in the Wild-Type and dyc283 Cells. Supplemental Movie 1. Time-Lapse Movie of the EB1c-33FLAGGFP Fusion in Two Cytokinetic Cells. Supplemental Movie Legends. ACKNOWLEDGMENTS We thank members of the Liu laboratory for critical input in the project. Our gratitude goes to Bruno Favery at Institut National de la Recherche Agronomique in France for the dyc283 mutant, Tobias Baskin at the University of Massachusetts, Amherst for the rsw7 mutant, Kazuo Shinozaki and Motoaki Seki at the Plant Science Center of the RIKEN Yokohama Institute in Japan for providing the cDNA clone, Tsuyoshi Nakagawa at Shimane University in Japan for the pGWB vectors, and Steven Backues and Sebastian Bednarek at the University of Wisconsin-Madison for the DRP1A antibody. We especially want to thank Li Tao and Jon Scholey for their advice on protein biochemistry and the use of their fast protein liquid chromatography system and John Jordan at Olympus USA for his assistance with the FV1000 confocal microscope. This report is based on work supported by the National Science Foundation under the grant MCB-0920454. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. T.H. was a Katherine Esau postdoctoral fellow. AUTHOR CONTRIBUTIONS C.-M.K.H and B.L. designed the research. C.-M.K.H, T.H., F.G., R.W.R., and Y.-R.J.L performed the research and analyzed data. C.-M.K.H, T.H., and B.L. wrote the article together. B.L. revised the article. Received July 23, 2010; revised June 28, 2011; accepted August 4, 2011; published August 26, 2011. References Ambrose, J.C., and Cyr, R. (2007). The kinesin ATK5 functions in early spindle assembly in Arabidopsis. Plant Cell 19: 226–236. Ambrose, J.C., Li, W., Marcus, A., Ma, H., and Cyr, R. (2005). A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis. Mol. Biol. Cell 16: 1584–1592. Andersland, J.M., Fisher, D.D., Wymer, C.L., Cyr, R.J., and Parthasarathy, M.V. (1994). Characterization of a monoclonal antibody prepared against plant actin. Cell Motil. Cytoskeleton 29: 339–344. Asada, T., Sonobe, S., and Shibaoka, H. (1991). Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350: 238–241. Austin II, J.R., Seguı́-Simarro, J.M., and Staehelin, L.A. (2005). Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J. Cell Sci. 118: 3895–3903. Bajer, A.S., Smirnova, E.A., and Mole-Bajer, J. (1993). Microtubuleconverging centers-implications for microtubule dynamics in higher plants. In Chromosome Segregation and Aneuploidy, B.K. Vig, ed (Berlin: Springer-Verlag), pp. 225–239. Bannigan, A., Scheible, W.-R., Lukowitz, W., Fagerstrom, C., Wadsworth, P., Somerville, C., and Baskin, T.I. (2007). A conserved role for kinesin-5 in plant mitosis. J. Cell Sci. 120: 2819–2827. Bednarek, S.Y., and Falbel, T.G. (2002). Membrane trafficking during plant cytokinesis. Traffic 3: 621–629. Bisgrove, S.R., Lee, Y.-R.J., Liu, B., Peters, N.T., and Kropf, D.L. (2008). The microtubule plus-end binding protein EB1 functions in root responses to touch and gravity signals in Arabidopsis. Plant Cell 20: 396–410. Caillaud, M.-C., Lecomte, P., Jammes, F., Quentin, M., Pagnotta, S., Andrio, E., de Almeida Engler, J., Marfaing, N., Gounon, P., Abad, P., and Favery, B. (2008). MAP65-3 microtubule-associated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis. Plant Cell 19: 423–437. Chan, J., Calder, G., Fox, S., and Lloyd, C. (2005). Localization of the microtubule end binding protein EB1 reveals alternative pathways of spindle development in Arabidopsis suspension cells. Plant Cell 17: 1737–1748. Eleftheriou, E.P., Baskin, T.I., and Hepler, P.K. (2005). Aberrant cell plate formation in the Arabidopsis thaliana microtubule organization 1 mutant. Plant Cell Physiol. 46: 671–675. Euteneuer, U., and McIntosh, J.R. (1980). Polarity of midbody and phragmoplast microtubules. J. Cell Biol. 87: 509–515. Gaillard, J., Neumann, E., Van Damme, D., Stoppin-Mellet, V., Ebel, C., Barbier, E., Geelen, D., and Vantard, M. (2008). Two microtubuleassociated proteins of Arabidopsis MAP65s promote antiparallel microtubule bundling. Mol. Biol. Cell 19: 4534–4544. Guan, K.L., and Dixon, J.E. (1991). Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192: 262–267. Hepler, P.K., and Jackson, W.T. (1968). Microtubules and early stages of cell-plate formation in the endosperm of Haemanthus katherinae Baker. J. Cell Biol. 38: 437–446. Hiwatashi, Y., Obara, M., Sato, Y., Fujita, T., Murata, T., and Hasebe, M. (2008). Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens. Plant Cell 20: 3094–3106. Hush, J.M., Wadsworth, P., Callaham, D.A., and Hepler, P.K. (1994). Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J. Cell Sci. 107: 775–784. Jiang, C.-J., and Sonobe, S. (1993). Identification and preliminary characterization of a 65 kDa higher-plant microtubule-associated protein. J. Cell Sci. 105: 891–901. Jürgens, G. (2005). Cytokinesis in higher plants. Annu. Rev. Plant Biol. 56: 281–299. Kang, B.H., Busse, J.S., Dickey, C., Rancour, D.M., and Bednarek, S.Y. (2001). The arabidopsis cell plate-associated dynamin-like protein, ADL1Ap, is required for multiple stages of plant growth and development. Plant Physiol. 126: 47–68. Kapitein, L.C., Janson, M.E., van den Wildenberg, S.M., Hoogenraad, C. C., Schmidt, C.F., and Peterman, E.J.G. (2008). Microtubule-driven multimerization recruits ase1p onto overlapping microtubules. Curr. Biol. 18: 1713–1717. Kawamura, E., Himmelspach, R., Rashbrooke, M.C., Whittington, A. T., Gale, K.R., Collings, D.A., and Wasteneys, G.O. (2006). MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol. 140: 102–114. Komaki, S., Abe, T., Coutuer, S., Inzé, D., Russinova, E., and Phragmoplast Microtubule Organization Hashimoto, T. (2010). Nuclear-localized subtype of end-binding 1 protein regulates spindle organization in Arabidopsis. J. Cell Sci. 123: 451–459. Kong, Z., Hotta, T., Lee, Y.R., Horio, T., and Liu, B. (2010). The g-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell 22: 191–204. Lauber, M.H., Waizenegger, I., Steinmann, T., Schwarz, H., Mayer, U., Hwang, I., Lukowitz, W., and Jürgens, G. (1997). The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139: 1485–1493. Lee, Y.-R.J., Giang, H.M., and Liu, B. (2001). A novel plant kinesinrelated protein specifically associates with the phragmoplast organelles. Plant Cell 13: 2427–2439. Lee, Y.R.J., and Liu, B. (2000). Identification of a phragmoplastassociated kinesin-related protein in higher plants. Curr. Biol. 10: 797–800. Lee, Y.R.J., Li, Y., and Liu, B. (2007). Two Arabidopsis phragmoplastassociated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell 19: 2595–2605. Liu, B., Joshi, H.C., and Palevitz, B.A. (1995). Experimental manipulation of g-tubulin distribution in Arabidopsis using anti-microtubule drugs. Cell Motil. Cytoskeleton 31: 113–129. Liu, B., Joshi, H.C., Wilson, T.J., Silflow, C.D., Palevitz, B.A., and Snustad, D.P. (1994). g-Tubulin in Arabidopsis: Gene sequence, immunoblot, and immunofluorescence studies. Plant Cell 6: 303–314. Lucas, J.R., Courtney, S., Hassfurder, M., Dhingra, S., Bryant, A., and Shaw, S.L. (2011). Microtubule-associated proteins MAP65–1 and MAP65–2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 23: 1889–1903. Mao, G.J., Chan, J., Calder, G., Doonan, J.H., and Lloyd, C.W. (2005). Modulated targeting of GFP-AtMAP65-1 to central spindle microtubules during division. Plant J. 43: 469–478. Müller, S., Smertenko, A., Wagner, V., Heinrich, M., Hussey, P.J., and Hauser, M.T. (2004). The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr. Biol. 14: 412–417. Nakagawa, T., Kurose, T., Hino, T., Tanaka, K., Kawamukai, M., Niwa, Y., Toyooka, K., Matsuoka, K., Jinbo, T., and Kimura, T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. Otegui, M., and Staehelin, L.A. (2000). Syncytial-type cell plates: A novel kind of cell plate involved in endosperm cellularization of Arabidopsis. Plant Cell 12: 933–947. Otegui, M.S., Verbrugghe, K.J., and Skop, A.R. (2005). Midbodies and phragmoplasts: Analogous structures involved in cytokinesis. Trends Cell Biol. 15: 404–413. Pan, R., Lee, Y.R.J., and Liu, B. (2004). Localization of two homologous Arabidopsis kinesin-related proteins in the phragmoplast. Planta 220: 156–164. Pesquet, E., and Lloyd, C. (2011). Microtubules, MAPs and xylem formation. In The Plant Cytoskeleton, B. Liu, ed (New York: Springer), pp. 277–306. Sasabe, M., Kosetsu, K., Hidaka, M., Murase, A., and Machida, Y. (2011). Arabidopsis thaliana MAP65-1 and MAP65-2 function redun- 2923 dantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signal. Behav. 6: 743–747. Sasabe, M., Soyano, T., Takahashi, Y., Sonobe, S., Igarashi, H., Itoh, T.J., Hidaka, M., and Machida, Y. (2006). Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 20: 1004–1014. Seki, M., Carninci, P., Nishiyama, Y., Hayashizaki, Y., and Shinozaki, K. (1998). High-efficiency cloning of Arabidopsis full-length cDNA by biotinylated CAP trapper. Plant J. 15: 707–720. Seki, M., et al. (2002). Functional annotation of a full-length Arabidopsis cDNA collection. Science 296: 141–145. Smertenko, A., Saleh, N., Igarashi, H., Mori, H., Hauser-Hahn, I., Jiang, C.-J., Sonobe, S., Lloyd, C.W., and Hussey, P.J. (2000). A new class of microtubule-associated proteins in plants. Nat. Cell Biol. 2: 750–753. Smertenko, A.P., Kaloriti, D., Chang, H.Y., Fiserova, J., Opatrny, Z., and Hussey, P.J. (2008). The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes. Plant Cell 20: 3346–3358. Smertenko, A.P., Chang, H.Y., Wagner, V., Kaloriti, D., Fenyk, S., Sonobe, S., Lloyd, C., Hauser, M.T., and Hussey, P.J. (2004). The Arabidopsis microtubule-associated protein AtMAP65-1: Molecular analysis of its microtubule bundling activity. Plant Cell 16: 2035–2047. Staehelin, L.A., and Hepler, P.K. (1996). Cytokinesis in higher plants. Cell 84: 821–824. Tao, L., and Scholey, J.M. (2010). Purification and assay of mitotic motors. Methods 51: 233–241. Van Damme, D., Bouget, F.Y., Van Poucke, K., Inzé, D., and Geelen, D. (2004a). Molecular dissection of plant cytokinesis and phragmoplast structure: A survey of GFP-tagged proteins. Plant J. 40: 386–398. Van Damme, D., Van Poucke, K., Boutant, E., Ritzenthaler, C., Inzé, D., and Geelen, D. (2004b). In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 136: 3956– 3967. van de Meene, A.M., Hohmann-Marriott, M.F., Vermaas, W.F., and Roberson, R.W. (2006). The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 184: 259–270. Vantard, M., Levilliers, N., Hill, A.M., Adoutte, A., and Lambert, A.M. (1990). Incorporation of Paramecium axonemal tubulin into higher plant cells reveals functional sites of microtubule assembly. Proc. Natl. Acad. Sci. USA 87: 8825–8829. Yoneda, A., Akatsuka, M., Hoshino, H., Kumagai, F., and Hasezawa, S. (2005). Decision of spindle poles and division plane by double preprophase bands in a BY-2 cell line expressing GFP-tubulin. Plant Cell Physiol. 46: 531–538. Zhang, D.H., Wadsworth, P., and Hepler, P.K. (1990). Microtubule dynamics in living dividing plant cells: Confocal imaging of microinjected fluorescent brain tubulin. Proc. Natl. Acad. Sci. USA 87: 8820–8824. Zhu, C.J., Lau, E., Schwarzenbacher, R., Bossy-Wetzel, E., and Jiang, W. (2006). Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc. Natl. Acad. Sci. USA 103: 6196–6201. Interaction of Antiparallel Microtubules in the Phragmoplast Is Mediated by the Microtubule-Associated Protein MAP65-3 in Arabidopsis Chin-Min Kimmy Ho, Takashi Hotta, Fengli Guo, Robert W. Roberson, Yuh-Ru Julie Lee and Bo Liu Plant Cell 2011;23;2909-2923; originally published online August 26, 2011; DOI 10.1105/tpc.110.078204 This information is current as of June 14, 2017 Supplemental Data /content/suppl/2011/08/10/tpc.110.078204.DC1.html /content/suppl/2011/08/18/tpc.110.078204.DC2.html References This article cites 53 articles, 32 of which can be accessed free at: /content/23/8/2909.full.html#ref-list-1 Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY