* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Chem Biology
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Bottromycin wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein moonlighting wikipedia , lookup
Genetic code wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein adsorption wikipedia , lookup
Expanded genetic code wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
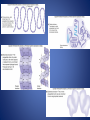
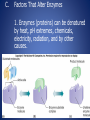
Organic Chemistry Compounds that contain both hydrogen and carbon are called organic, the others are inorganic. Organic Substances: 1. Carbohydrates a. Carbohydrates provide primary source of energy for cellular activities and are composed of carbon, hydrogen, and oxygen. b. Carbohydrates are made from monosaccharides (simple sugars); disaccharides are two monosaccharides joined together; complex carbohydrates polysaccharides), such as starch, are built of many sugars. 2. Lipids: a. Lipids are insoluble (waterproof) in water and include fats (waxes and oils), phospholipids, and steroids. b. Fats supply long term energy storage, are composed of oxygen, carbon, and hydrogen, and are built from glycerol and three fatty acids. c. Fatty acids with hydrogen at every position along the carbon chain are saturated; those with one or more double bonds are called unsaturated fats. c. Phospholipids contain glycerol, two fatty acids, and a phosphate group, and are important in biological membranes. d. Steroids are complex ring structures, and include cholesterol, which is used to synthesize the sex hormones (estrogen, progesterone, testosterone). • To form fats, glycerol and fatty acids bond. 3. Proteins: a. Proteins have a great variety of functions in the body---build tissues, as certain hormones, as receptors on cell membranes, as antibodies to fight disease, and as enzymes to catalyze metabolic reactions and cell processes. b. Proteins contain C, O, H, and nitrogen atoms; some also contain sulfur. c. Building blocks of proteins are the amino acids, each of which has a carboxyl group, an amino group, and a side chain called the R group. d. The bonds between amino acids in polypeptides are called peptide bonds. e. Proteins have complex shapes held together by hydrogen bonds. f. Protein shapes (conformation), which determine how proteins function, can be altered (denatured) by pH, temperature, radiation, or chemicals. • The bond between two amino acids is a peptide bond; two bound amino acids form a dipeptide, while many joined form a polypeptide. Denaturing • When hydrogen bonds break in a protein as a result of exposure to heat, radiation, pH changes, or exposure to chemicals. 4. Nucleic Acids: a. Nucleic acids form genes and take part in protein synthesis. b. They contain carbon, hydrogen, oxygen, nitrogen, and phosphorus, which are bound into building blocks called nucleotides. c. Nucleic acids are of two major types: DNA (with deoxyribose) and RNA (with ribose). d. RNA (ribonucleic acid) functions in protein synthesis; DNA (deoxyribonucleic acid) stores the molecular code in genes. Enzymes A. Enzymes control the rates of all the metabolic reactions of the cell. B. Enzyme Action 1. Enzymes are complex proteins that function to lower the activation energy of a reaction so it may begin and proceed more rapidly. Enzymes are called catalysts. 2. Enzymes work in small quantities and are recycled by the cell. 3. Each enzyme is specific, acting on only one kind of substrate. 4. Active sites on the enzyme combine with the substrate and a reaction occurs. 5. The speed of enzymatic reactions depends on the number of enzyme and substrate molecules available. • Enzymes are typically named for the substrate and then end in –ASE • Example: The enzyme that would work on the substrate “sucrose” would be named “sucrase” C. Factors That Alter Enzymes 1. Enzymes (proteins) can be denatured by heat, pH extremes, chemicals, electricity, radiation, and by other causes. Lock and Key Analogy