* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Projections of the amygdala to the thalamus in the cynomolgus
Feature detection (nervous system) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroplasticity wikipedia , lookup
Affective neuroscience wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Emotional lateralization wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Basal ganglia wikipedia , lookup
Limbic system wikipedia , lookup
THE JOURNAL OF COMPARATIVE NEUROLOGY 222:56-68 (1984)
Projections of the Amygdala to the Thalamus
in the Cynomolgus Monkey
J.P. AGGLETON
AND
M. MISHKIN
Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda,
Maryland 20205
ABSTRACT
The projections of the amygdala to the thalamus in cynomolgus monkeys (Mucaca fascicularis) were studied with both anterograde and retrograde axonal tracing techniques. Horseradish peroxidase (HRP) was injected into medial and midline thalamic sites in five animals, and tritiated
amino acids were injected into selected amygdaloid regions in a total of 13
hemispheres in ten animals. The findings from the two types of tracer experiments demonstrated the origins, course, and terminal pattern of amygdaloid
projections to two thalamic nuclei -medialis dorsalis (MD)and reuniens. Almost all of the amygdaloid nuclei contribute projections to MD, though the
greatest proportion arise from the basal group and terminate in discrete, interlocking patches within the medial, magnocellular portion of MD. In addition to this major projection, the central and medial amygdaloid nuclei send
a lighter projection to the lateral portion of nucleus reuniens. The amygdalothalamic projections took a variety of routes out of the amygdala before the
large majority joined the inferior thalamic peduncle and entered the rostral
head of the thalamus where they turned caudally toward their targets.
A small number of amygdalothalamic fibers may also run in the stria
terminalis.
Key words: amygdala, thalamus, monkey
The projections from the amygdala to the thalamus are
currently of great interest for several reasons. First, both
regions appear to possess important mnemonic functions.
Thus, bilateral temporal-lobe damage involving the amygdala and hippocampus and damage to the medial, periventricular regions of the thalamus have both been consistently associated with profound amnesic syndromes
(Scoville and Milner, '57; Victor et al., '71; Brierley, '77).
Furthermore, there is evidence that the medial thalamic region that has been implicated in diencephalic amnesia, specifically the nucleus medialis dorsalis (Victoret d., '71; McEntee et al., '76; Markowitsch, '82), receives projections
from the amygdala and not from the hippocampus. Second, recent research has indicated that both the amygdala
and parts of the medial and midline thalamus are rich in
opiate receptors (Kuhar et al., '73; Wamsley et al., '82). In
fact the anatomical connections in question link two of the
regions with the highest concentration of opiate receptors
in the primate brain. Last, amygdaloid efferents to the medial thalamus provide a potentially important route by
which a limbic structure may indirectly influence large regions of prefrontal cortex (Nauta, '72; Porrino et al., '81),
this cortex being the major target of nucleus medialis
dorsalis.
Evidence of direct projections from the amygdala to the
0 1984 ALAN R. LISS, INC.
thalamus in primates was first provided by the studies of
Fox ('49)and Nauta ('61). These authors described degeneration in the magnocellular portion of nucleus medialis
dorsalis following lesions in the amygdala of the rhesus
monkey. Additional degeneration was noted in nucleus reuniens, nucleus centralis inferior, and nucleus paracentralis (Nauta, '61), as well as in the medial pulvinar (Fox,
'49). These earlier studies, however, could provide only a
limited description of the amygdaloid projection system,
and interpretation of the origin of the system was confounded by the possible existence of thalamic projections
from the adjacent allocortex, areas which may have been
directly damaged or disconnected in the course of the
amygdaloid surgery.
Accepted July 29, 1983.
John Aggleton's present address is Department of Psychology,
University of Durham, Science Laboratories, South Road,
Durham, DH1 3LE England.
A preliminary report of these data was presented at the 12th
Annual Meeting of the Society for Neuroscience (Aggleton and
Mishkin, '82).
Address reprint requests to M. Mishkin at Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda,
MD 20205.
AMYGDALOTHALAMIC PROJECTIONS IN MONKEY
The newer axonal transport methods for both anterograde and retrograde tracing provide a sensitive means by
which the precise amygdalothalamic connections may be
detailed. We therefore used the retrograde transport of
horseradish peroxidase (HRP) to determine the origin of
the thalamic connections within the amygdala, while the
thalamic projections of the individual amygdaloid nuclei
were investigated with autoradiography.
MATERIALS AND METHODS
The subjects were 15 cynomolgus monkeys (Macacafascicularis) weighing 2.5-7.5 kg at the time of surgery. The
animals were tranquilized with ketamine hydrochloride (10
mgkg), anesthetized with Nembutal (35 mgkg), and
placed in a stereotaxic apparatus.
Five monkeys received injections of horseradish peroxidase into various medial thalamic regions. Under sterile
precautions, bone and dural flaps were opened above the
most dorsal portion of the central sulcus. The wall of the
left hemisphere was then retracted and a 5-10-mm portion
of the corpus callosum was split longitudinally to expose
the thalamic midline (massa intermedia). The coordinates
for the injections were determined from the positions of
the third ventricle at either end of the mama intermedia.
In three animals injections of 40% HRP (Sigma, type VI)
were delivered through a 1-plHamilton syringe at a rate of
0.01 pl/3 minutes. In two of these animals (A3 and A5) a
total of 0.15 pl of HRP was injected into the nucleus medialis dorsalis and the posterior thalamic midline, while in
the third (A2)0.13 pl of HRP was injected into the anterior
thalamic midline. In an additional animal (A26)0.8 pl of a
4% solution of HRP (Sigma, type VI) conjugated with
wheat germ agglutin was injected into the anterior thalamus. In the final animal in the series (A7),a 10% solution
of HRP in TRIS buffer was delivered iontophoretically
with a glass pipette into nucleus medialis dorsalis.
Two days after the surgery the monkeys with HRP injections were deeply anesthetized with Nembutal and perfused intracardially with 0.9% saline followed by a solution of 1% paraformaldehyde and 1.25% glutaraldehyde in
0.1 M phosphate buffer (pH 7.2).The brains were stored in
30% sucrose buffer at pH 7.2 at 4°C for 3-4 days and then
cut in 50-pm coronal sections, and a one-in-fiveseries was
reacted with tetramethyl benzidine (TMB) according to
the protocols of either Hardy and Heimer (’77)or Mesulam
(’78).Alternate reacted sections were dehydrated, counterstained with thionine, and coverslipped, whereas the remainder were simply dehydrated and coverslipped. The
57
sections were studied with both brightfield and darkfield
optics.
In the ten other cynomolgus monkeys an equal-parts
mixture of tritiated proline (New England Nuclear, L-[2,3,
4, 5 3H],specific activity 139 Cilmmole),and leucine (New
England Nuclear, L-[3, 4, 5 3H], specific activity 111 Ci/
mmole), was injected into the amygdala at a final concentration of 50 &i/pl. The injection coordinates were determined by reference to internal skull landmarks revealed on
x-rays (Aggleton and Passingham, ’81). A 1-pl Hamilton
syringe was lowered vertically into the amygdaloid region
and between 0.1 and 1.0 pl of the amino acids was injected.
Unilateral injections were made in seven monkeys (A4,A6,
A10, A13, A16, A17, A18) and bilateral injections were
made in the remainder (A20,A21, A22),for a total of 13 injection sites. After an interval of 6 or 7 days the monkeys
were deeply anesthetized with Nembutal and perfused intracardially with buffered 10% formol saline. The brains
were cut in 33-pmcoronal sections on a freezing microtome
and every sixth section was mounted from either phosphate buffer or Perfix and coated with Kodak NTB2 emulsion. The sections were exposed at 4°C for between 2 and
30 weeks, with one series from every animal being exposed
for at least 20 weeks. The sections were developed in
Kodak D19, fixed, and counterstained with thionine.
RESULTS
Cyt oarchit ecture
The lack of a standardized nomenclature for the amygdaloid nuclei (Price, ’81a)makes it necessary to choose among
the schemes proposed by various investigators. The classification adopted in this study (Fig. 1)is based on the cytoarchitectural descriptions of the primate amygdala by
Crosby and Humphrey (’41).
Figure 1illustrates the relative positions of the amygdaloid nuclei. Five “deep” nuclei may be distinguished,
namely, the lateral, lateral basal, medial basal, accessory
basal, and central nuclei. There is, in addition, a poorly defined “anterior amygdaloid area”which lies below the endopiriform nucleus (Krettek and Price, ’78)and between the
central and medial nuclei (Fig. la,b).The cytoarchitectural
characteristics of these “deep” nuclei have been detailed
previously (see Crosby and Humphrey, ’41; Lauer, ’45,
Jiminez-Castellanos, ’49). Although there is no absolute
border between the lateral basal and medial basal nuclei
they are treated as distinct, a viewpoint supported by evidence of connectional differences (HerzogandVan Hoesen,
’76; Pandya et al., ’81).
Abbreviations
A
AC
ACC B
AHA
AM
AV
BNST
Ct
Cdc
CE
CL
c1
Clc
CnMd
CTA
Anterior amygdaloid area
Anterior commissure
Accessory basal nucleus
Amygdalohippocampal area
Nucleus anterior medialis
Nucleus anterior ventralis
Bed nucleus of stria terminalis
Tail of caudate
Nucleus centralis densocellularis
Central nucleus
Claustrum
Nucleus centralis lateralis
Nucleus centralis latocellularis
Nucleus centrum medianum
Cortical transition area
EC
END
F
GP
H
IC
ITP
LAT
LB
MB
ME
MD
MDmc
MDpc
oc
External capsule
Endopiriform nucleus
Fornix
Globus pallidus
Hippocampus
Internal capsule
Inferior thalamic peduncle
Lateral nucleus
Lateral basal nucleus
Medial basal nucleus
Medial nucleus
Nucleus medialis dorsalis
Pars magnocellularis
Pars parvocellularis
Optic chiasm
OT
P
Pa
PAM
Pcn
Pf
R
Re
SI
SM
ST
TMT
VA
VAmc
X
Optic tract
Putamen
Nucleus paraventricularis
Periamygdaloid cortex
Nucleus paracentralis
Nucleus parafascicularis
Nucleus reticularis
Nucleus reuniens
Substantia innominata
Stria medullaris
Stria terminalis
Mammillothalamic tract
Nucleus ventralis anterior
Pars magnocellularis
Area X
58
J.P. AGGLETON AND M. MISHKIN
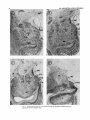
Fig. 1. Coronal sections taken at 1-mm intervals through the amygdaloid complex showing the
major nuclear divisions (thionine stain).
AMYGDALOTHALAMIC PROJECTIONS IN MONKEY
The superficial nuclei of the amygdala, which lie on the
medial surface of the temporal lobe, comprise the medial
and cortical nuclei, the periamygdaloid cortex, and the corticoamygdaloid transition area (Fig. 1).The medial nucleus
is composed predominantly of small to medium multipolar
neurons which are most densely packed near the medial
edge of the nucleus. The cortical nucleus lies ventral to the
medial nucleus and medial to the accessory basal nucleus,
with which it has no clear border (Fig. lb,c). Ventral and
anterior to the cortical nucleus lies the periamygdaloid cortex, which is distinguished from the cortical nucleus by its
greater preponderance of pyramidal cells and a wider,
more uniform layer I11 (Fig. 1).Located between the periamygdaloid cortex and the entorhinal cortex, and medial
to the medial basal nucleus, is a region of pyramidal-type
cells which show almost no signs of lamination. This is the
corticoamygdaloid transition area, which is found along
the caudal two-thirds of the amygdala. In addition to these
areas, a transitional “amygdalohippocampal area”lying between the amygdala and the hippocampus at the caudal extreme of the amygdala can also be identified (Fig. Id).
The nomenclature for the thalamus is taken from the description of Olszewski (’52).This classification, which is
based on the cytoarchitectural appearance of the thalamus
of the rhesus monkey (Macaca mulatta),is equally applicable to the cynomolgus monkey. Two thalamic nuclei, medialis dorsalis (MD) and reuniens, are considered in this
study. Nucleus medialis dorsalis, which occupies much of
the posterior medial quadrant of the thalamus, may be divided into four subareas: pars magnocellularis, parvocellularis, multiformis, and densocellularis. The medial portion
of MD, pars magnocellularis (MDmc),is identified by its
large, well-spaced spherical neurons. Nucleus reuniens,
one of the midline nuclei, extends along the ventral surface
of the massa intermedia, lying adjacent and dorsal to the
third ventricle. This nucleus is composed of small, heterogeneous neurons.
HRP experiments
Amygdaloid HRP label. Figure 2 illustrates the extent
of the thalamic injections in four of the five brains studied.
The figure depicts the region around the injection site in
which the HRP reaction product is distinguishable under
brightfield conditions. Three monkeys (A3, A5, A7) received injections which involved almost the entire extent
of the magnocellular portion of nucleus medialis dorsalis.
These injections always involved, in addition, the adjacent
nuclei paraventricularis, centralis superior, centralis inferior, centralis intermedialis, centralis densocellularis, and
the medial aspect of the parvocellular portion of medialis
dorsalis (Fig. 3). In one case (A3)the injection site was centered in the caudal midline thalamic nuclei and extended
into the medial aspect of nucleus parafascicularis, the
most caudal portion of nucleus reuniens, the medial habenula, and much of nucleus medialis dorsalis, while in the
other two cases (A5, A7) some HRP leaked into the transected corpus callosum.
These three experiments, which produced very similar
patterns of amygdaloid label, provided evidence of a moderate amygdalothalamic projection system. The findings
in monkey A5, which were typical, are illustrated in Figure
4. Cells labelled with HRP were found throughout the
amygdala, but the greatest concentration always occurred
in the medial basal nucleus. This nucleus contained approximately one-half of the total HRP-positive cells in the
59
amygdala of animals A3, A5, and A7. Labelled cells were
found throughout the medial basal nucleus (Fig. 4).though
there was a tendency for them to be concentrated in its medial half. The lateral basal nucleus contained the second
highest number of HRP-positive cells in these three monkeys, though there were considerably fewer than in the medial basal nucleus. A small number of labelled cells was
found in the accessory basal nucleus, the periamygdaloid
cortex, and the corticoamygdaloid transition area. Only occasional labelled cells were seen in the remaining amygdaloid nuclei. There was no evidence that the HRP-positive
cells were concentrated in any particular cytoarchitectural
subdivision within the amygdaloid nuclei.
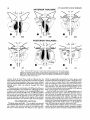
HRP was injected into the anterior thalamic midline in
two animals (A2, A26). In both cases, the HRP spread
throughout nuclei anterior medialis, centralis densocellularis, centralis latocellularis, alaris, rotundus, and the rostral half of nucleus paraventricularis but not into nucleus
medialis dorsalis (Fig. 2).
In animal A26 the injection extended ventrally to include all of the anterior portion of nucleus reuniens and the
most dorsal portion of the dorsomedial hypothalamic nucleus. Numerous cells labelled with HRP were found
throughout the dorsal amygdala in this case. The greatest
concentrations of these labelled cells occurred in the medial nucleus and in the medial portion of the central nucleus. The remaining amygdaloid nuclei contained only a
handful of labelled cells, with an occasional cell being present in each amygdaloid nucleus (Fig. 4). A similar anterior
thalamic injection was placed in animal A2, though the
injection extended ventrally only to the dorsal margin of
nucleus reuniens. In this case, there was only a very small
accumulation of labelled cells in the central and medial nuclei and in the amygdalohippocampal area, and a total of
only one or two cells each were found in most of the other
amygdaloid nuclei.
Cells labelled with HRP were also found in a variety of
sites adjacent to the amygdala (Fig. 4).The piriform, entorhinal, prorhinal, and perirhinal cortices (Van Hoesen and
Pandya, ’75) all contained significant numbers of labelled
cells following the injections centered in MD (A3, A5, A7).
These cortical cells, which were mainly pyramidal, were located in layers I11 and V. The substantia innominata contained retrogradely labelled cells in all animals. This label
was particularly dense in animal A26, in which the anterior
thalamic midline was injected.
Well-defined anterograde HRP label was observed only
in the amygdala of animal A26 (Fig. 2). The anterograde label in this case was present throughout much of the complex, with the greatest concentration appearing in the lateral basal and medial nuclei and in the medial portion of
the central nucleus. In contrast, the lateral and accessory
basal nuclei and the lateral portion of the central nucleus
contained little anterograde label.
Course of amygdalothalamic connections. Labelled fibers running between the amygdala and the thalamus
were observed in several animals (A3, A5, A26). Although
it was impossible to determine whether these fibers contained anterograde or retrograde label, the relative scarcity of anterograde label within the amygdala makes it
likely that the direction of the majority of the fibers is
amygdalothalamic. The fibers typically took one of two
routes through the amygdala. One group of fibers could be
seen either cutting across the face of the rostral amygdala
or running in the external capsule adjacent to the lateral
J.P. AGGLETON AND M. MISHKIN
60
ANTERIOR THALAMIC
$el
0
A2
‘‘C
A2
A26
Q
A26
a2
A26
A5
A7
POSTERIOR THALAMIC
I
(
I
A7
A5
A7
Fig. 2. Extent of HRP injections in anterior (A2, A26) and posterior (A5, A7) medial thalamus,
mapped onto standard coronal sections at three different rostrocaudal levels. The coronal thalamic
sections have been split a t the midline and depict the hemispheres containing the greatest spread of
HRP. Areas in black represent the region in which the underlying cytoarchitecture is completely
masked by the HRP reaction product.
nucleus. Some of these fibers could be followed into the
white matter between the rhinal sulcus and the amygdala
and into the ventral amygdala. These sets of axons merged
in the substantiainnominata with the other major group of
labelled axons, which ran directly through the dorsal
amygdala.
The presence of a small number of HRP-positive fibers in
the stria terminalis in two animals (A5,A26) suggests that
at least a small component of the amygdalothalamic connections may travel in this fiber tract. In addition, labelled
fibers were seen running in the external capsule between
the claustrum and the putamen in case A5. These fibers
could be followed ventrally into the region of the anterior
perforated substance, just dorsal to the rostral amygdala.
Autoradiographic experiments
Thalamic amino acid label. The amygdala was injected
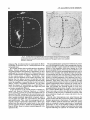
with tritiated amino acids in a total of 13hemispheres. Figure 5 depicts the locus and extent of the injection sites.
The size of each injection was defined as the region in
which an appreciable accumulation of silver grains could
be observed within the perikaryon after a development period of at least 6 weeks. Each of the amygdaloid nuclei,
with the exception of the anterior area, was involved in at
least one case. There was extraamygdaloid spread in only
three injections: into the head of the hippocampus in one
(A13),into the entorhinal cortex in another (AlO),and into
both of these regions in the third (A4).
Terminal label was found in the magnocellular part of
MD (Fig. 6) in those cases in which the injection was centered in either the medial basal nucleus (A4, A10, A13),or
the lateral basal nucleus (A6, A21-left, A21-right),or the
accessory basal nucleus (A18, A20-left, A2O-right),or the
ventral portion of the lateral nucleus (A16).In contrast, injections involving the dorsal portion of the lateral nucleus
(A22-right),the central nucleus (A17),or the medial nucleus (A22-left)did not result in label within MD.
The thalamic label in MDmc always occurred in patches
which were often interlinked, thereby forming a complex
reticular pattern. There was little overlap between the tha-
AMYGDALOTHALAMIC PROJECTIONS IN MONKEY
Fig. 3. Brightfield photomicrograph of injection site in animal A5
(thionine stain).
61
served in the midline and anterior thalamic nuclei in A4
and A13, there was spread of the isotope into the rostral
head of the hippocampus in both cases, and the pattern of
thalamic label matched that seen after injections entirely
confined to the hippocampus (Rosene and Van Hoesen, '77;
Aggleton and Mishkin, '82).
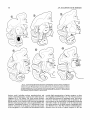
Course of amygdaloid efferents. The route of the
amygdaloid projections to the thalamus could be traced
back through the inferior thalamic peduncle to the ventral
amygdalofugal pathway. Fibers leaving the amygdala
took either a direct dorsal or an indirect ventral route (Fig.
8, section 1) before joining the ventral amygdalofugal
pathway. These latter fibers left the amygdala ventrally to
join the white matter between the medial basal nucleus
and the rhinal sulcus. The efferents then passed either anteriorly around the rostral face of the amygdala or laterally
and then dorsally to run in the most medial edge of the external capsule, adjacent to the lateral nucleus. The different sets of ventrally exiting fibers appeared to converge
above the dorsal amygdala with the majority of efferents
that passed directly out of the dorsal amygdala, where
they combined to form the ventral amygdalofugal pathway (Fig. 8, sections 1, 2). The proportion of fibers leaving
the amygdala by the ventral route appeared greatest in
those cases in which the injection was centered in the medial basal or lateral basal nuclei.
Label in the ventral amygdalofugal pathway could be
seen passing below the anterior commissure through the
substantia innominata and the most ventral portion of globus pallidus before rising medial to the anterior limb of the
internal capsule to reach the bed nucleus of the stria terminalis (Fig. 8, sections 2 and 3). Some of the fibers in the
more caudal portions of the ventral amygdalofugal pathway were seen to join the inferior thalamic peduncle and
ascend above the posterior hypothalamus to enter the rostral pole of the thalamus. These amygdaloid efferents
formed diffuse bundles which entered the head of the thalamus and passed medial to nucleus reticularis before running caudally through the medial, magnocellular portion
of ventralis anterior (Fig. 8, section 4). These fiber bundles
then crossed nucleus paracentralis and entered the head of
medialis dorsalis where they fanned caudally and dorsally
to terminate within the magnocellular portion of the nucleus (Fig. 8, sections 5 and 6).
In all cases, with the exception of A22-right,transported
label was also carried in the stria terminalis. Although labelled fibers were not observed to branch off the stria and
join the thalamus directly, it is possible that some amygdalothalamic fibers run the length of the stria to its bed
nucleus before turning caudally to enter the thalamus. Unfortunately, the close proximity of the stria terminalis
fibers entering the bed nucleus with those in the inferior
thalamic peduncle (Fig. 8, sections 2,3) made it impossible
to determine whether or not the stria terminalis does contribute directly to the amygdalothalamic projection. Finally, other amygdaloid efferents could be seen entering
the external capsule and rising dorsal and medial to the
claustrum (Fig. 8, all sections). It was not possible to determine, however, whether any of these fibers terminated in
the thalamus.
lamic terminal fields of the different cases, indicating that
the projections from the various amygdaloid nuclei terminate in distinct subareas of MDmc (Fig. 7). In all cases the
projection to MDmc was concentrated within the rostral
two-thirds of the nucleus. Given the apparent topography
of the projections, however, the absence of label in caudal
MDmc could reflect a failure to inject the appropriate
locus in the amygdala. Injections centered in the lateral
and accessory basal nucleus produced the lightest terminal label observed in MDmc (A16, A18, A20-left, A20right). An amygdaloid projection to the contralateral
MDmc was observed, but only in the animals with the
largest amygdaloid injections (A4, A6). This projection always fell within the same loci as the ipsilateral one but was
considerably lighter and smaller.
Light terminal label was found in the ipsilateral portion
of nucleus reuniens (Fig. 7)in animals A17 and A22-left following injections involving primarily the central and medial nuclei, respectively. This label, which was particularly
light in the animal with an injection in the medial nucleus
(A22-left),was restricted to the rostral half of nucleus reuniens just ventral to nucleus centralis latocellularis. The
projection was confined to the lateral portion of nucleus reuniens, an area distinguished from the medial portion by
its rounder, more deeply staining cells. No label was found
DISCUSSION
in MD in either of these cases.
The present study has confirmed the existence of amygThere was no evidence of a projection to any other thalamic nucleus in those cases in which the injection was re- daloid efferents originating in the basolateral group of
stricted to the amygdala. Although terminal label was ob- amygdaloid nuclei that terminate in nucleus medialis dor-
J.P. AGGLETON A N D M. MISHKIN
62
ANTERIOR THALAMIC HRP INJECTION
u
POSTERIOR THALAMIC HRP INJ ECTlON
Fig. 4. Distribution of HRP-positive cells mapped onto standard coronal sections a t three rostrocaudal levels of the amygdala, following injections centered in the anterior thalarnic midline (upper
row) and nucleus medialis dorsalis (lower row). Each dot represents one labelled cell.
salis of the thalamus (Nauta, '61). In addition, the HRP
and autoradiographic experiments revealed the contribution of the individual amygdaloid nuclei and permitted
comparisons between the projections of the various amygdaloid regions. The findings indicate that the projection to
MD is highly organized, with the efferents from the various amygdaloid nuclei terminating in different subareas of
this nucleus. Evidence was also found of a lighter thalamic
projection arising in the central and medial nuclei and terminating in nucleus reuniens. A similar projection from
just the central nucleus has been described by Price and
Amaral ('81).The routes of these thalamic projections were
traced in both the retrograde and anterograde transport
experiments.
Organization of amygdalothalamic projections
Injections of HRP centered in nucleus medialis dorsalis
revealed amygdaloid projections arising predominantly
from the basal group of nuclei. The medial basal nucleus
provided the majority of this input, with the lateral and accessory basal nuclei supplying a smaller but significant
contribution. The autoradiographic experiments demonstrated that these amygdaloid efferents project to the
magnocellular portion of nucleus medialis dorsalis
(MDmc),with the various amygdaloid subdivisions terminating in different portions of the nucleus. Thus, the lateral amygdala was found to project to anteroventral
MDmc, the lateral basal region to the central core of
MDmc, and the accessory and medial basal nuclei to the
more dorsal portions of MDmc. A light projection to the
contralateral MDmc was also observed in the animals with
the largest amygdaloid injections. Finally, the concordance between the HRP and autoradiographic experiments indicates that little if any HRP was transported to
the amygdala by fibers of extrathalamic origin running
through the injection site. On the other hand, it is possible
that some of the retrograde amygdaloid label found after
HRP injections into the anterior thalamic midline originated from amygdaloid fibers projecting through the injection site to MDmc. However, the markedly different
pattern of amygdaloid HRP label that resulted from injections in the anterior as compared with the posterior medial
AMYGDALOTHALAMIC PROJECTIONS IN MONKEY
ANT.
MID.
63
POST.
Fig. 5. Distribution of amino acid injections throughout the rostrocaudal extent of the amygdala.
White numerals refer to separate cases, and 1 and r refer to left and right hemispheres, respectively.
Case APO-right is not shown as it largely overlaps with A2O-left.
J.P. AGGLETON AND M. MISHKIN
64
Fig. 6. Darkfield (left)and hrightfield (right)photomicrographs showing termination of amygdalaid projection within the magnocellular portion of nucleus medialis dorsalis in an animal with a large
amino acid injection centered in nucleus lateralis basalis (A6).The apparent label in stria medullaris
(asterisk)is artifactual.
thalamus (Fig. 4 ) indicates that any such uptake by fibers
of passage can account only for a small proportion of the
retrograde label.
It is unclear whether the amygdala projects throughout
MDmc. Discrete injections of amino acids within the
amygdala produced small patches of termination, as first
mentioned by Price and Amaral ('81).These patches were
found to interlock with one another and thereby fill much
of the rostral portion of MDmc. Furthermore, these
patches were less apparent in the largest injections, suggesting that the spaces between the patches had been
filled in. As the present series of injections did not totally
cover the amygdala, it is possible that the entire extent of
MDmc receives amygdaloid projections. On the other
hand, the absence of terminations in caudal MDmc in
every one of the cases suggests that this region may well
not receive amygdaloid efferents.
A light projection to the rostral portions of nucleus reuniens was observed following injections of tritiated
amino acid that involved the central and medial nuclei. In
line with this, HRP was placed in the rostral thalamic midline in two animals, but only the injection that involved nucleus reuniens produced appreciable retrograde label in
the amygdala; this label was concentrated in the medial
and central nuclei. Thus, both the anterograde and retrograde transport experiments demonstrate a direct projection from the central and medial nuclei to the rostral
portion of nucleus reuniens. Unlike the amygdaloid projections to MDmc, those to nucleus reuniens appeared to be
ipsilateral only.
The amygdalothalamic projections followed two routes
out of the amygdaloid nucleus. Some amygdaloid efferents
coursed ventrally to pass around the anterior and lateral
borders of the amygdala, while others passed out of the
dorsal amygdala directly. These two sets of efferents combined to form the ventral amygdalofugal pathway, part of
which sweeps medially and caudally through the substantia innominata and then rises dorsally to pass behind the
anterior commissure into the bed nucleus of the stria terminalis. A caudal component of this pathway joins the inferior thalamic peduncle and rises into the rostral tip of the
thalamus. These latter fibers then run caudally through
the magnocellular portion of nucleus ventralis anterior and
nucleus paracentralis before entering the rostral pole of
MD. In addition, there was evidence that the stria terminalis, and possibly the external capsule as well, carry
amygdalothalamic projections, though it was never possible to follow labelled fibers through the entire length of
these tracts and so confirm these alternative routes. Last,
no evidence could be found of a thalamic projection in the
stria medullaris, though such a route may exist in the rat
(Heimer, '72).
Several minor discrepancies were noted between the
present results and those of previous studies of amygdalothalamic projections in the primate. For example, no conclusive evidence could be found from either the anterograde or retrograde transport experiments of amygdaloid
projections to any of the thalpmnic midline nuclei other
than nucleus reuniens, though such projections have been
reported following an injection of tritiated amino acids
AMYGDALOTHALAMICPROJECTIONS IN MONKEY
65
ANTERIOG
THALAMUS
\
VA
I
Clc
POSTERIOR THALAMUS
+6.9 0
A10
Fig. 7. Termination patterns of thalamic afferents arising from different amygdaloid regions.
These projections have been mapped onto four coronal sections: the numerals refer to comparable sections in the atlas of Olszewski ('52).
into the caudal portion of the central nucleus (Price and
Amaral, '81).These additional projections would appear to
be very light, however, as there was no evidence of an accumulation of HRP-positive cells in the central nucleus in
cases A2 and A3, in which the injections were located in
the anterior and posterior midline thalamic nuclei, respectively. Also, a much larger number of amygdalothalamic
projections than described here was suggested by Porrino
et al. ('81).though it is evident that in the latter study this
was due, at least in part, to the spread of amino acids into
the adjacent temporal cortex and hippocampus. Finally,
no evidence could be found of a projection to the medial
pulvinar (Fox, '49; Price and Amaral, '81).
The only other species in which amygdalothalamic projections have been studied systematically is the rat, and
the overall pattern of the projections in this species appears to match closely those in the monkey. As in the monkey, nucleus medialis dorsalis in the rat is the major thalamic target of the amygdaloid complex (Krettek and
Price, '77). This projection arises from the basolateral
nucleus and the endopiriform nucleus and terminates, respectively, in the medial and central portions of MD. There
is also some evidence that the projections from the basolateral nucleus have a crude topography, but, unlike the
case in the monkey, these projections in the rat have a rostrocaudal organization and do not appear to terminate in
distinctive patches. Finally, there is a report of a light
amygdaloid projection to nucleus reuniens which arises
predominantly from the medial nucleus and the anterior
area (Herkenham, '78). However, the failure to find evidence of similar thalamic afferents in the cat (Krettek and
Price, '77) indicates that interspecies variability in amygdalothalamic projections may be much greater than that
found thus far between rat and monkey.
Relationship of amygdalothalamic projections
to other amygdaloid connections
While the majority of amygdaloid projections to the
forebrain are reciprocated (Aggleton et al., '80; Price, '81b),
the amygdalothalamic connections provide a clear exception. Most amygdaloid efferents to the thalamus terminate in MDmc, yet it has been shown repeatedly that thalamic projections to the amygdala arise not from MDmc
but from some of the midline nuclei, from some of the intra-
J.P. AGGLETON AND M. MISHKIN
66
n
Fig. 8. Coronal sections illustrating the pattern of labelled fibers, shown in dashes, emerging from
a representative amygdaloid injection of amino acids (animal A6). Successive sections are approximately 2 mm apart. This figure depicts the major efferent routes from the amygdala into the ventral
amygdalofugal pathway, the stria terminalis. and the external capsule. The locus of fibers in the stria
terminalis is indicated by dense dashes, both ventrally and dorsally, in sections 3-6. The termination
of the thalamic projection in MDmc is shown dotted.
laminar nuclei including nucleus parafascicularis and
nucleus subparafascicularis, and from the medial pulvinar
(Aggleton et al., ’80; Mehler, ’80). Only nucleus reuniens
appears to possess reciprocal amygdaloid connections, the
lateral portion of this nucleus both receiving amygdaloid
efferents and projecting back to the amygdala (Mehler,’80;
Aggleton, unpublished results). I t is interesting to note,
however, that most of the thalamic nuclei that do project
to the amygdala (i.e., the midline and intralaminar nuclei)
contain high concentrations of opiate receptors, as does
the amygdala itself (Wamsley et al., ’82).This concordance
may reflect the existence of “opiatergic tracts” that course
both ways between the amygdala and the thalamus. Recent evidence on the distribution of enkephalincontaining
cell bodies within the amygdala of the rat indicates that
the central nucleus is the major source of opiatergic efferents (Roberts et al., ’82). This evidence combined with indications that the levels of opiate receptors in MD are
__
AMYGDALOTHALAMIC PROJECTIONS IN MONKEY
lower than in the adjacent midline and intralaminar nuclei
(Wamsley et al., '82) suggests that the projection from the
amygdala to nucleus medialis dorsalis is not part of the
putative opiatergic system.
Nauta ('62, '72) has already noted that the existence of
amygdalothalamic projections provide an indirect route
by which the amygdala may influence prefrontal cortex.
The medial, magnocellular portions of MD which receive
amygdaloid afferents project, in turn, upon the orbital and
medial surfaces of the prefrontal cortex (Pribram et al., '53;
Tobias, '75; Kievit and Kuypers, '77). In addition, direct
amygdaloid projections to the prefrontal cortex have been
demonstrated, these projections terminating especially
within the orbital prefrontal, medial prefrontal, and anterior cingulate cortices (Porrinoet d.,'81; Price, '81b).These
direct amygdaloprefrontal projections arise from the basal
group of nuclei, the same nuclei that provide the majority
of the efferents to nucleus medialis dorsalis. Thus, the
same amygdaloid region may project both directly and indirectly through MDmc to the orbital and medial prefrontal regions. Furthermore, the organized pattern of the
amygdalothalamic projections suggests that the different
amygdaloid nuclei may be indirectly linked with different
regions of prefrontal cortex. Neither the direct nor indirect
amygdaloprefrontal projections have yet been detailed,
however, and so it is unclear whether or not these two projection systems are congruent.
Amygdalothalamic projections and memory
An indicated earlier, chronic anterograde amnesia in
man is associated with damage in two regions -the medial
temporal lobe and the medial diencephalon. The existence
of projections from the hippocampus and amygdala to the
medial diencephalon through the fornix and inferior thalamic peduncle, respectively, indicates that the medial
temporal and medial thalamic regions may constitute an
interconnected system subserving memory. Indirect evidence of the importance of the inferior thalamic peduncle
for memory processes is provided by the frequent failure of
fornix lesions alone to produce a notable degree of amnesia
in man, a finding which suggests that the remaining medial temporal-diencephalic pathway can support mnemonic functions. Furthermore, nucleus medialis dorsalis,
to which the amygdala but not the hippocampus projects,
is the thalamic structure most frequently implicated in diencephalic amnesia (Victor et al., '71; Markowitsch, '82).
The notion that amygdalothalamic projections are part
of a temporodiencephalic memory system is also supported by neuropsychological studies in monkeys. Thus,
combined ablation of the amygdala and the hippocampus
results in a far more severe memory loss than does ablation
of either structure alone (Mishkin, '78). Similarly, combined disconnection of these two structures from the diencephalon yields a much more severe memory loss than does
disconnection of either structure alone (Bachevalier et al.,
'82). Finally, combined destruction of the major thalamic
targets of the amygdala and the hippocampus, namely,
MDmc and the anterior nuclei, yields a more severe amnesia than does destruction of either target alone (Aggleton
and Mishkin, '83a,b). In short, there is now both clinical
and experimental evidence that both the amygdalothalamic projections and the hippocampodiencephalic projections are part of an integrated system for memory
(Mishkin, '82). By confirming that MDmc is the main thalamic target of the amygdala, the present experiments
67
strengthen the conclusion that this nucleus has an important mnemonic role. This, in turn, suggests the intriguing
but still untested possibility that the areas to which
MDmc projects, namely, the medial and orbital prefrontal
cortex, may likewise play an important role in memory.
ACKNOWLEDGMENTS
We thank J.N. Sewell, L.G. Ungerleider, and D.P. Freidman for all their assistance.
LITERATURE CITED
Aggleton, J.P., M.J. Burton, and R.E. Passingham (1980)Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 190:347-368.
Aggleton, J.P., and M. Mishkin (1982) A comparison of amygdaloid and
bippocampal projections to the thalamus in monkeys. Sac. Neurosci.
Abstr. 8:240.6.
Aggleton. J.P., and M. Mishkin (1983a)Visual recognition impairment following medial thalamic lesions in monkeys. Neuropsychologia 2k189197.
Aggleton. J.P., and M. Mishkin (1983b)Memory impairments following restricted medial thalamic lesions in monkeys. Exp. Brain Res. (in press).
Aggleton, J.P., and R.E. Passingham (1981) Stereotaxic surgery under Xray guidance in the rhesus monkey. with special reference t o the amygdala. Brain Res. 44.271-276.
Bachevalier, J., J.K. Parkinson, J.P. Aggleton, and M. Mishkin (1982) Severe recognition impairment after combined but not separate transection of the fornix and the amygdalofugal pathways. Sac. Neurosci.
Ahstr. 8:11.5.
Brierley, J.B. (1977)Neuropathology of amnesic states. In C.W.M. Whitty
and O.L. Zangwill (eds):Amnesia. London: Butterworths, pp. 199-223.
Crosby. E.C., and T. Humphrey (1941)Studies of the vertebrate telencephalon. 11. The nuclear pattern of the anterior olfactory nuclei, tuberculum olfactorium. and the amygdaloid complex in adult man. J. Camp.
Neurol. 74:309-352.
Fox, C.A. (1949)Amygdalo-thalamic connections inMacaca mulutta. Anat.
Rec. 103:537-538.
Hardy, H., and L. Heimer (1977) A safer and more sensitive substitute for
diaminobenzidene in the light microscopic demonstration of retrograde
and anterograde axonal transport of HRP. Neurosci. Letters 5:235240.
Heimer, L. (1972)The olfactory connections of the diencephalon in the rat.
Brain Behav. Evol. 6:484-523.
Herkenham, M. (1978) The connections of nucleus reuniens thalami: Evidence for a direct thalamo-hippocampal pathway in the rat. J. Camp.
Neural. 177:589-610.
Herzog, A.G.. and G.W. Van Hoesen (1976) Temporal neocortical afferent
connections of the amygdala in the rhesus monkey. Brain Res. 115:
57-69.
Jimenez-Castellanos, J. (1949)The amygdaloid complex in monkey studied
by reconstructional methods. J. Camp. Neural. 91:507-526.
Kievit, J., and H.G.J.M. Kuypers (1977) Organization of thalamocortical
connexions to the frontal lobe of the rhesus monkey. Exp. Brain Res. 29:
299-322.
Krettek, J.E.. and J.L. Price (1977) Projections from the amygdaloid complex to the cerebral cortex and the thalamus in the rat and cat. J. Camp.
Neural. 172.687-722.
Krettek, J.E., and J.L. Price (1978) A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid connections. J. Comp. Neurol. 178:255-280.
Kuhar, M.J., C.B. Pert, and S.H. Snyder (1973)Regional distribution of opiate receptor binding in monkey and human brain. Nature 245447-450.
Lauer, E.W. (1945) The nuclear pattern and fiber connections of certain
basal telencephalic centers in the macaque. J. Camp. Neurol. 82:215253.
Markowitsch, H.J. (1982) Thalamic mediodorsal nucleus and memory: A
critical evaluation of studies in animals and man. Neurosci. Biobehav.
Rev. 6:351-380.
McEntee, W.J., M.P. Biber, D.P. Perl. and D.F. Benson (1976)Diencephalic
amnesia: A reappraisal. J. Neural. Neurosurg. Psychiatr. 39:436-441.
Mehler, W.R. (1980) Subcortical afferent connections of the amygdala in
the monkey. J. Camp. Neural. 190:733-762.
68
Mesulam, M.-M. (1978) Tetramethyl benzidene for horseradish peroxidase neurohistochemistry: A non carcinogenic blue reaction-product
with superior sensitivity for visualizing neural afferents and efferents.
J. Histochem. Cytochem. 26:106-117.
Mishkin, M. (1978)Memory in monkeys severely impaired by combined but
not by separate removals of the amygdala and hippocampus. Nature
273:297-298.
Mishkin, M. (1982)A memory system in the monkey. Philos. Trans. R. SOC.
Lond. [Biol.]298:85-95.
Nauta, W.J.H. (1961) Fibre degeneration following lesions of the amygdaloid complex in the monkey. J. Anat. 95:515-530.
Nauta, W.J.H. (1962)Neural associations of the amygdaloid complex in the
monkey. Brain 85:505-520.
Nauta, W.J.H. (1972) Neural associations of the frontal cortex. Acta Neurobiol. Exp. 32:125-140.
Olszewski, I. (1952)The Thalamus of the Macaca mulatta. Basel: S . Karger.
Pandya, D.N.. G.W. Van Hoesen, and M.M. Mesulam (1981)Efferent connections of the cingulate gyms in the rhesus monkey. Exp. Brain Res.
42:319-330.
Porrino, L.J., A.M. Crane, and P.S. Goldman-Rakic (1981)Direct and indirect pathways from the amygdala to the frontal lobe in the rhesus monkey. J. Comp. Neurol. 198:121-136.
Pribram, K.H., K.L. Chow, and J. Semmes (1953)Limit andorganizationof
the cortical projection from the medial thalamic nucleus in monkey. J.
Comp. Neurol. 98:433-448.
Price, J.L. (1981a) Toward a consistent terminology for the amygdaloid
J.P. AGGLETON AND M. MISHKIN
complex. In Y. Ben-Ari (ed): The Amygdaloid Complex. New York:
Elsevier, pp. 13-18.
Price, J.L. (1981b)The efferent projections of the amygdaloid complex in
the rat, cat and monkey. In Y. Ben-Ari (ed):The Amygdaloid Complex.
New York: Elsevier, pp. 121-132.
Price, J.L., and D.G. Amaral (1981) An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci.
1:1242-1259.
Roberts, G.W., P.L. Woodhams, J.M. Polak, andT.J. Crow (1982)Distribution of neuropeptides in the limbic system of the rat: The amygdaloid
complex. Neuroscience 7:99-13 1.
Rosene. D.L., and G.W. Van Hoesen (1977) Hippocampal efferents reach
widespread areas of cerebral cortex and amygdala in rhesus monkey.
Science 198:315-317.
Scoville, W.B.. and B. Milner (1957) Loss of recent memory after bilateral
hippocampal lesions. J. Neurol. Neurosurg. Psychiatr. 2O:ll-21.
Tobias, T.J. (1975)Afferents t o prefrontal cortex from the thalamic niediodorsal nucleus in the rhesus monkey. Brain Res. 83:191-212.
Van Hoesen, G.E., and D.N. Pandya (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. 1.
Temporal lobe afferents. Brain Res. 951-24.
Victor, M.. R.D. Adams. and G.H. Collins (1971) The Wernicke-Korsakoff
Syndrome. Oxford: Blackwells.
Wamsley. J.K., M.A. Zarbin, W.S. Young, and M.J. Kubar (1982)Distribution of opiate receptors in the monkey brain: An autoradiographic
study. Neuroscience 7:595-613.