* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
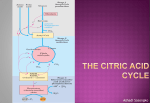
Download Lecture 28 - Citrate Cycle
Biochemical cascade wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Metalloprotein wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup