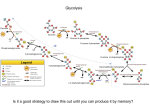
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Citrate Cycle
Multi-state modeling of biomolecules wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Citrate Cycle Lecture 28 Key Concepts • The Citrate Cycle captures energy using redox reactions • Eight reactions of the Citrate Cycle • Key control points in the Citrate Cycle regulate metabolic flux • What role does NADH and FADH2 have in connecting the citrate cycle to ATP synthesis? • Why is the citrate cycle considered the hub of metabolism? The citrate cycle is considered the "hub" of cellular metabolism because it not only links the oxidation of metabolic fuels (carbohydrate, fatty acids and proteins) to ATP synthesis, but it also provides shared metabolites for numerous other metabolic pathways. Overview of the Citrate Cycle • • • • • • The citrate cycle captures energy using redox reactions Acetyl-CoA, derived from pyruvate (from glycolysis), is the “normal” entry substrate into the cycle Pyruvate can also be derived from amino acid catabolism, and acetyl-CoA can be derived from both amino a acids and fatty acids. Large enzyme complexes are involved As name states, it is a cyclical pathway “Products” of citrate cycle are CO2, NADH, FADH2 and GTP All of the enzymes in the citrate cycle, electron transport chain and oxidative phosphorylation reside in the mitochondrial matrix where pyruvate is converted to acetyl-CoA by the enzyme pyruvate dehydrogenase. Function of the Citrate Cycle • • • Primary function : oxidize acetyl-CoA Transfer four pairs of electrons from citrate cycle intermediates to 3 moles of NADH and 1 mole of FADH2 during each turn of the cycle It is a "metabolic engine" in which all eight of the cycle intermediates are continually replenished to maintain a smooth-running energy conversion process – The fuel is acetyl-CoA – The exhaust is two molecules of CO2 – The energy output is eight electrons (redox energy) and one GTP that is converted to ATP (phosphoryl transfer energy). Redox energy is used to generate ATP • "currency exchange" for redox energy and ATP synthesis in the mitochondria – for every 2 NADH molecules that are reoxidized in the electron transport chain, ~5 molecules of ATP are produced by oxidative phosphorylation (~2.5 ATP/ NADH). – For every 2 FADH2 molecules that are reoxidized by the electron transport chain, only ~3 molecules of ATP are produced (~1.5 ATP/FADH2) • Why the difference? – differences in where these two coenzymes enter the electron transport chain. • Based on ATP currency exchange ratio, and the one substrate level phosphorylation reaction, each turn of the cycle produces ~10 ATP for every acetyl-CoA that is oxidized. • Since regeneration of NAD+ and FAD inside the mitochondrial matrix is required to maintain flux through the citrate cycle (four of the enzymes require NAD+ or FAD as coenzymes), this metabolic engine is dependent on a constant supply of O2, just like a combustion engine. Pathway overview Other names for citrate cycle Hans Krebs, a biochemist who fled Nazi Germany for England in 1933, first described the citrate cycle in 1937. The citrate cycle is sometimes called the Krebs cycle or the tricarboxylic acid cycle (citrate is a tricarboxylate) or the citric acid cycle, although we will refer to it as the citrate cycle. Considering that the pKa values of the three carboxylate groups in citric acid are 3.1, 4.7 and 6.4, why is "citrate cycle" a better term than "citric acid cycle" or "tricarboxylic acid cycle"? Pathway Questions What does the citrate cycle accomplish for the cell? – Transfers 8 electrons from acetyl-CoA to the coenzymes NAD+ and FAD to form 3 NADH and 1 FADH2 which are then re-oxidized by the electron transport chain to produce ATP by the process of oxidative phosphorylation. – Generates 2 CO2 as “waste products” and uses substrate level phosphorylation to generate 1 GTP which is converted to ATP by nucleoside diphosphate kinase. – Supplies metabolic intermediates for amino acid and porphyrin biosynthesis. Pathway Questions What is the overall net reaction of citrate cycle? Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2 H2O → CoA + 2 CO2 + 3 NADH + 2 H+ + FADH2 + GTP ∆Gº’ = -57.3 kJ/mol Pathway Questions What are the key regulated enzymes in citrate cycle? Pyruvate dehydrogenase – not a citrate cycle enzyme but it is critical to flux of acetyl-CoA through the cycle; this multisubunit enzyme complex requires five coenzymes, is activated by NAD+, CoA and Ca2+ (in muscle cells), and inhibited by acetyl-CoA, ATP and NADH. Citrate synthase – catalyzes the first reaction in the pathway and can be inhibited by citrate, succinyl-CoA, NADH and ATP; inhibition by ATP is reversed by ADP. Pathway Questions What are the key regulated enzymes in citrate cycle? Isocitrate dehydrogenase - catalyzes the oxidative decarboxylation of isocitrate by transferring two electrons to NAD+ to form NADH, and in the process, releasing CO2, it is activated by ADP and Ca2+ and inhibited by NADH and ATP. α-ketoglutarate dehydrogenase - functionally similar to pyruvate dehydrogenase in that it is a multisubunit complex, requires the same five coenzymes and catalyzes an oxidative decarboxylation reaction that produces CO2, NADH and succinyl-CoA; it is activated by Ca2+ and AMP and it is inhibited by NADH, succinylCoA and ATP. Pathway Questions What are examples of citrate cycle in real life? Citrate is produced commercially by fermentation methods using the microorganism Aspergillus niger. Every year almost a half of million tonnes (5 x 108 kg) of citrate are produced worldwide by exploiting the citrate synthase reaction. The complete oxidation of glucose to CO2 and H2O is summarized by the reaction: Glucose (C6H12O6) + 6O2 → 6CO2 + 6H2O ∆Gº’ = -2,840 kJ/mol ∆G = -2,937 kJ/mol Citrate cycle is where most of this CO2 is produced Where does the rest of it come from? 8 Reactions of the Citrate Cycle In the first half of the cycle, the two carbon acetate group of acetyl-CoA is linked to the four carbon oxaloacetate substrate to form a six carbon citrate molecule. Citrate is then transformed to isocitrate to set up two decarboxylation reactions yielding two NADH and the high energy four carbon cycle intermediate succinyl-CoA. In the second half of the cycle, oxaloacetate is regenerated from succinylCoA by four successive reactions that lead to the formation of one GTP (ATP), one FADH2 and one NADH. 8 Reactions of the Citrate Cycle Reaction 1: Condensation of oxaloacetate and acetyl-CoA by citrate synthase to form citrate Purpose: commit the acetate unit of acetyl-CoA to oxidative decarboxylation Reaction follows an ordered mechanism: 1. oxaloacetate binds, inducing a conformational change in the enzyme that facilitates: 2. acetyl-CoA binding 3. formation of the transient intermediate, citryl-CoA 4. rapid hydrolysis that releases CoA-SH and citrate Reaction 2: Isomerization of citrate by aconitase to form isocitrate reversible two step isomerization reaction the intermediate, cis-aconitate, is formed by a dehydration reaction that requires the participation of an iron-sulfur cluster (4Fe-4S) in the enzyme active site water is added back to convert the double bond in cis-aconitate to a single bond with a hydroxyl group on the terminal carbon Aconitase is one of the targets of fluorocitrate Fluorocitrate is derived from fluoroacetate. Fluoroacetatecontaining plants, such as acacia found in parts of Australia and Africa, are so deadly that Australian sheep herders have reported finding sheep with their heads still in the bush they were feeding on when they died. Reaction 3: Oxidative decarboxylation of isocitrate by isocitrate dehydrogenase to form α-ketoglutarate, CO2 and NADH First of two decarboxylation steps in the citrate cycle First reaction to generate NADH used for energy conversion reactions in the electron transport system Catalyzes an oxidation reaction that generates the transient intermediate oxalosuccinate In the presence of the divalent cations Mg2+ or Mn2+, oxalosuccinate is decarboxylated to form α-ketoglutarate Reaction 4: Oxidative decarboxylation of by α-ketoglutarate dehydrogenase to form succinyl-CoA, CO2 and NADH Second oxidative decarboxylation reaction (produces NADH) α-Ketoglutarate dehydrogenase complex utilizes essentially the same catalytic mechanism we have already described for the pyruvate dehydrogenase reaction Includes the binding of substrate to an E1 subunit (α-ketoglutarate dehydrogenase), followed by decarboxylation and formation of a TPP-linked intermediate Reaction 5: Conversion of succinyl-CoA to succinate by succinyl-CoA synthetase in a substrate level phosphorylation reaction that generates GTP The available free energy in the thioester bond of succinyl-CoA (∆Gº' = -32.6 kJ/mol) is used in the succinyl-CoA synthetase reaction to carry out a phosphoryl transfer reaction (∆Gº' = +30.5 kJ/mol) that leads to the production of GTP (or ATP) and succinate. Nucleoside diphosphate kinase interconverts GTP and ATP by a readily reversible phosphoryl transfer reaction: GTP + ADP ↔ GDP + ATP (∆Gº' = 0 kJ/mol). Reaction 6: Oxidation of succinate by succinate dehydrogenase to form fumarate This coupled redox reaction directly links the citrate cycle to the electron transport system through the redox conjugate pair FAD/FADH2 which is covalently linked to the enzyme succinate dehydrogenase, an inner mitochondrial membrane protein. Oxidation of succinate results in the transfer of an electron pair to the FAD moiety, which in turn, passes the two electrons to the electron carrier coenzyme Q in complex II of the electron transport system. Reaction 7: Hydration of fumarate by fumarase to form malate Fumarase is a highly stereo-specific enzyme Catalyzes the reversible hydration of the C=C double bond in fumarate to generate the L-isomer of malate. Stereospecificity of enzymes is an important feature that we will see again and again in metabolism. Enantiomers are Mirror Images Also called Stereoisomers COO- COO- HO H H OH H H H H COO- COO- L-malate L-Dogbert D-malate Plane of Reflection D-Dogbert Reaction 8: Oxidation of malate by malate dehydrogenase to form oxaloacetate Final reaction in the citrate cycle (Yeah!) Oxidation in the presence of NAD+ of the hydroxyl group of malate to form oxaloacetate Change in standard free energy for this reaction is unfavorable (∆Gº' = +29.7 kJ/mol) Extremely low concentration of oxaloacetate inside the mitochondrial matrix ensures that the reaction is pulled to the right under physiological conditions (as a result of actual free energy, ∆G). Bioenergetics of the citrate cycle Glycolysis + pyruvate dehydrogenase reaction + citrate cycle = net reaction: Glucose + 2 H2O + 10 NAD+ + 2 FAD + 4 ADP + 4 Pi → 6 CO2 + 10 NADH + 6 H+ + 2 FADH2 + 4 ATP Reducing power of 10 NADH and 2 FADH2 can be converted to ATP equivalents using the currency exchange ratios of ~2.5 ATP/NADH and ~1.5 ATP/FADH2 to yield ~28 ATP, which when combined with the 4 ATP synthesized by substrate phosphorylation, generates a maximum of ~32 ATP. The complete oxidation of glucose by the pyruvate dehydrogenase complex and the citrate cycle leads to the production of 6 CO2 molecules as “waste”. Use of radioactively-labeled metabolic intermediates such as 14C-acetyl CoA What happens to the two carbon atoms that enter the citrate cycle as acetylCoA? In the first turn of the cycle, both carbons are incorporated into oxaloacetate. It isn't until the second cycle that the first carbon is released as CO2 by the isocitrate dehydrogenase reaction. The second carbon is not lost as CO2 until the α-ketoglutarate reaction in the fourth turn of the cycle. → Intermediates must be continually regenerated in a cyclic pathway to maintain metabolic flux. We now do this with 13C labeled compounds as well. Regulation Of The Citrate Cycle The three main control points: 1. citrate synthase 2. isocitrate dehydrogenase 3. α-ketoglutarate dehydrogenase Pyruvate dehydrogenase and pyruvate carboxylase control the rate of citrate cycle flux by providing acetyl CoA and oxaloacetate, respectively. These enzymes are also regulated.