* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Woodward–Hoffmann rules wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Aldol reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
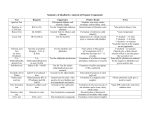
生科一甲 有機實驗下學期 EXPERIMENT I. Sodium Borohydride Reaction of Acetophenone Let us illustrate the mechanism of borohydride reduction using acetophenone the compound you will reduce in Experiment 15.1. The reduction of the carbonyl group takes place by the nucleophilic attack of BH4- on the carbon. In this reaction, the large amount of ethanol used as the solvent converts the product alkoxide to the product alcohol. EXPERIMENT II. Synthesis of Aspirin Experiment 16.3 is the esterification of salicylic acid, which contains a phenol group, with acetic anhydride. If the esterificaion were attempted with acetic acid, the yield of the ester would be negligible. EXPERIMENT III. Synthesis of Dibenzalacetone In acidic or basic solution, aldehydes can undergo condensations, reactions in which molecules combine to yield larger molecules. With aldehydes, this particular type of condensation reaction is called an aldol condensation, or an aldol addition, because the product is both an aldehyde and an alcohol. EXPERIMENT IV. A Grignard Reaction (Step 1) In this experiment, the Grignard reagent is prepared by slowly adding a solution of 1-bromobutane in anhydrous diethyl ether (not solvent ether, which is wet) to Mg turnings. (Step 2) The reaction of n-butylmagnesium bromide with acetone is extremely vigorous. The product is a magnesium alkoxide sometimes forms a crusty precipitate that must be broken up by swirling the flask so that the unreacted acetone can become mixed with the Grignard reagent. EXPERIMENT V. Qualitative Organic Analysis (A. DNP Test for Carbonyl Compound) The reaction of an aldehyde or ketone with 2,4-dinitrophenylhydrazin yields a precipitate of a 2,4- dinitrophenylhydrazin, or DNP. Alcohols and phenols do not undergo reaction with this reagent. (B. Ceric Nitrate Test for Alcohols and Phenols) Alcohols or phenols yield colored complex ions when treated with ceric ammonium nitrate. Other oxygen functional groups (aldehyde, ketone, carboxyl, etc.) do not interfere with the complex formation except as noted in the Limitations. (C. Schiff Test for Aldehydes) The Schiff test (also called the fuchsin-aldehyde test) is based upon the reaction of an aldehyde with a colorless form of a rosaniline dye to yield a violet or purple product. Ketones fail to undergo this reaction. (D. Tollens Test for Aldehydes) The Tollens test is the treatment of a sample with a solution containing silver-ammonia complex ions. When an aldehyde group is oxidized by this reagent, the silver ions are reduced to metallic silver, which forms a black precipitate and, if the test tube is clean, a silver mirror on the test tube. (E. Ferric Chloride Test for Phenols) When a phenol is treated with a solution of ferric chloride, colored complex ions are rapidly formed. Alcohols do not undergo this reaction; therefore, the test can be used to distinguish phenols from alcohols. (F. Alcohol Classification Test) The Lucas test is based upon the fact that primary, secondary, and tertiary alcohols vary in their rates of reaction with a mixture of conc.