element - Mrs. Phillips` Physical Science Webpage
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
Chemical Change
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
Chemistry Test Review – 8th Science Vocabulary: Element atom
... The difference between mass number and atomic mass The charge and location of each type of subatomic particle (proton, neutron, electron) Electrons Which ones have the most energy Which ones have the least energy Trends in the Periodic Table Atomic Radius Valence electrons Characteristics of Metals ...
... The difference between mass number and atomic mass The charge and location of each type of subatomic particle (proton, neutron, electron) Electrons Which ones have the most energy Which ones have the least energy Trends in the Periodic Table Atomic Radius Valence electrons Characteristics of Metals ...
Fall Final Exam Review Questions
... 40. Draw the Lewis Dot structures for the following: Potassium, Carbon, Iodine and Xenon? 41. What are properties of metals and where are they generally located on a periodic table? 42. What are properties of nonmetals and where are they generally located? 43. What are properties of metalloids and w ...
... 40. Draw the Lewis Dot structures for the following: Potassium, Carbon, Iodine and Xenon? 41. What are properties of metals and where are they generally located on a periodic table? 42. What are properties of nonmetals and where are they generally located? 43. What are properties of metalloids and w ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... (a) The diameter of an atom is less than the diameter of its nucleus. (b) The relative atomic mass of an atom is the mass of an atom relative to an atom of 12C. (c) p-orbitals can contain a maximum of 10 electrons. (d) The first ionisation energy of an element is the energy input (in kg mol-1) requi ...
... (a) The diameter of an atom is less than the diameter of its nucleus. (b) The relative atomic mass of an atom is the mass of an atom relative to an atom of 12C. (c) p-orbitals can contain a maximum of 10 electrons. (d) The first ionisation energy of an element is the energy input (in kg mol-1) requi ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
September 28th Notes
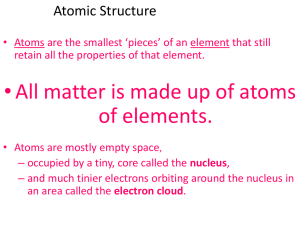
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
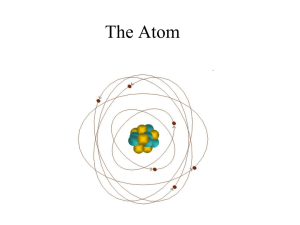
Atom
... Mendeleev- First Periodic Table ( 1869) • Grouped by atomic mass & chemical properties. • Blank spaces were left to add predicted elements ...
... Mendeleev- First Periodic Table ( 1869) • Grouped by atomic mass & chemical properties. • Blank spaces were left to add predicted elements ...
Chemistry Semester One Exam Review Name:
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
Bill Nye Atoms Workseet
... In Bill’s model, how far away is the electron from the nucleus? ______________________________________ If atoms are mostly empty space, how come they act like a solid?____________________________________ Atoms are the basic ____________________ _____________________ of all matter. With hydrogen in H ...
... In Bill’s model, how far away is the electron from the nucleus? ______________________________________ If atoms are mostly empty space, how come they act like a solid?____________________________________ Atoms are the basic ____________________ _____________________ of all matter. With hydrogen in H ...
Periodic Table of Elements * Study Guide
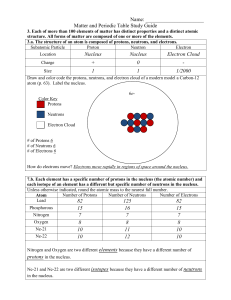
... Study and understand the following: Atomic Structure: How to find an element’s: atomic number atomic mass what two particles make up the atomic mass? what makes up the atom’s volume? # of protons Electrical charge of proton, electron, neutron # of electrons # of neutrons group # ...
... Study and understand the following: Atomic Structure: How to find an element’s: atomic number atomic mass what two particles make up the atomic mass? what makes up the atom’s volume? # of protons Electrical charge of proton, electron, neutron # of electrons # of neutrons group # ...
here
... 2. Describe how ionization energy and electronegativity change as you move from left to right along the periodic table and from top to bottom. Explain how all three of these plays a role in determining whether an atom “wants” to give away or take electrons. 3. Define what an atom’s atomic radius is. ...
... 2. Describe how ionization energy and electronegativity change as you move from left to right along the periodic table and from top to bottom. Explain how all three of these plays a role in determining whether an atom “wants” to give away or take electrons. 3. Define what an atom’s atomic radius is. ...
Salesian High School Elements and atoms Chemistry quiz The
... 1) The scientist who used alpha particles to bombard a thin film of gold, and proposed that most of the volume of an atom is empty space because most of the alpha particles went straight through the film: a) Rutherford b) Kelvin c) Thomson d) Dalton ...
... 1) The scientist who used alpha particles to bombard a thin film of gold, and proposed that most of the volume of an atom is empty space because most of the alpha particles went straight through the film: a) Rutherford b) Kelvin c) Thomson d) Dalton ...
What is Matter? Anything that can be smelled, tasted, touched… Has
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
What is Matter
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
Minerals * Chemistry Review
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
Atoms - misshoughton.net
... Each element has its own distinct properties. cannot be broken down into simpler parts by a chemical change. Compounds: pure substances made of more than one type of atom. Compounds are made of elements. NaCl (sodium chloride) is an example of a compound. ...
... Each element has its own distinct properties. cannot be broken down into simpler parts by a chemical change. Compounds: pure substances made of more than one type of atom. Compounds are made of elements. NaCl (sodium chloride) is an example of a compound. ...