ATOMS AND THE PERIODIC TABLE chapter three
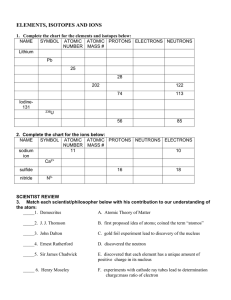
... – This makes the mass of different atoms of the same element different. – The average mass is a weighted number so that more common isotopes have a greater affect on the average than rare isotopes. ...
... – This makes the mass of different atoms of the same element different. – The average mass is a weighted number so that more common isotopes have a greater affect on the average than rare isotopes. ...
Atom through Periodic Table Study Guide
... 11. ..And then grouped that order into columns of elements with similar _____. 12. Moseley improved the P.T by putting the elements in order of increasing ________. 13. Imaginary element Floobium, Fo, has electrons in 5 energy levels. What period would it be in? 14. If imaginary Floobium has 6 valen ...
... 11. ..And then grouped that order into columns of elements with similar _____. 12. Moseley improved the P.T by putting the elements in order of increasing ________. 13. Imaginary element Floobium, Fo, has electrons in 5 energy levels. What period would it be in? 14. If imaginary Floobium has 6 valen ...
CHAPTER6_MEET_THE_ELEMENTS
... Rarely are metals found lying around in chunks in nature. Instead, they are almost mixed with other materials in compounds. This mixture, buried in rock is called a mineral and if there is enough of the mineral present, to make it worthwhile to mine, the rock is called an ore. Extraction - is the m ...
... Rarely are metals found lying around in chunks in nature. Instead, they are almost mixed with other materials in compounds. This mixture, buried in rock is called a mineral and if there is enough of the mineral present, to make it worthwhile to mine, the rock is called an ore. Extraction - is the m ...
The Periodic Table OL Page 1 of 2 G. Galvin Name: Periodic Table
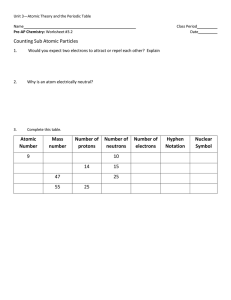
... -define atomic number (Z) and mass number (A) -define relative atomic mass (Ar) using 12C scale -define isotope -describe the composition of isotopes using hydrogen and carbon as an example -describe the organisation of particles in atoms of elements numbers 1-20 -classify the first twenty elem ...
... -define atomic number (Z) and mass number (A) -define relative atomic mass (Ar) using 12C scale -define isotope -describe the composition of isotopes using hydrogen and carbon as an example -describe the organisation of particles in atoms of elements numbers 1-20 -classify the first twenty elem ...
Ch. 2 note packet
... In a given compound, the relative numbers of atoms of each kind are definite and constant. In general, these relative numbers can be expressed as integers or simple fractions. IN GENERAL Elements consist of tiny particles called _________, which retain their identity in ____________________. In a co ...
... In a given compound, the relative numbers of atoms of each kind are definite and constant. In general, these relative numbers can be expressed as integers or simple fractions. IN GENERAL Elements consist of tiny particles called _________, which retain their identity in ____________________. In a co ...
periodic table
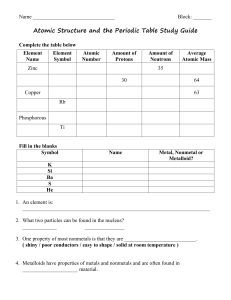
... Developed in the 1870’s by Mendeleyev with an increasing atomic # (# protons). Increasing Atomic mass would be incorrect, why? because of the isotopes of atoms (different masses). Organized and read like reading a book: across and down -Arranged in periods (rows) by increasing atomic # (what is at t ...
... Developed in the 1870’s by Mendeleyev with an increasing atomic # (# protons). Increasing Atomic mass would be incorrect, why? because of the isotopes of atoms (different masses). Organized and read like reading a book: across and down -Arranged in periods (rows) by increasing atomic # (what is at t ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 5. In Rutherford's Atomic model, the protons - positively charged particles are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The ...
... 5. In Rutherford's Atomic model, the protons - positively charged particles are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The ...
Atomic Models 2015-2016
... More about Elements.. • Elements are the building blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
... More about Elements.. • Elements are the building blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
2.1 The Nature of Matter - Sonoma Valley High School
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
answers
... d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
... d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
Exam 1 Review Questions
... Covalent compounds contain both metal and nonmetal atoms. Ionic compounds are made of molecules. Dmitri Mendeleev published the first modern atomic theory in 1805. Fluorine is found as a metal in its pure form. Francium chloride FrCl is a covalent compound. Graphite is a compound containing carbon a ...
... Covalent compounds contain both metal and nonmetal atoms. Ionic compounds are made of molecules. Dmitri Mendeleev published the first modern atomic theory in 1805. Fluorine is found as a metal in its pure form. Francium chloride FrCl is a covalent compound. Graphite is a compound containing carbon a ...
Review Notes - Biochemistry
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
Chemical reactions revision
... Elements are the building blocks of chemistry. Every element contains only one type of atom Each element contains atoms different to every other element Elements are arranged in the Periodic Table of elements. Element are arranged in the table in order of their atomic number Elements in different gr ...
... Elements are the building blocks of chemistry. Every element contains only one type of atom Each element contains atoms different to every other element Elements are arranged in the Periodic Table of elements. Element are arranged in the table in order of their atomic number Elements in different gr ...
Chem vocab quiz definitons
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
Chemistry Presentation: Part One
... • We can’t see the parts of atoms, even with modern technology, so we have an Atomic Theory • A theory is a good, logical idea about something but it hasn’t been proven to be true ...
... • We can’t see the parts of atoms, even with modern technology, so we have an Atomic Theory • A theory is a good, logical idea about something but it hasn’t been proven to be true ...
Power point on the Periodic Table
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...