October 26 AP Biology - John D. O`Bryant School of Math & Science
... Where did the glucose come from? Where did the O2 come from? Where did the CO2 come from? Where did the CO2 go? Where did the H2O come from? Where did the ATP come from? What else is produced that is not listed in this equation? ...
... Where did the glucose come from? Where did the O2 come from? Where did the CO2 come from? Where did the CO2 go? Where did the H2O come from? Where did the ATP come from? What else is produced that is not listed in this equation? ...
Biology 1407 - Ranger College
... - energy used to pump hydrogen (H+) from matrix into intermembrane space - H+ becomes more concentrated (100 times) in intermembrane space as in matrix - H+ concentration gradient drives chemiosmosis - chemiosmosis is process of producing ATP from ADP + P by an enzyme complex called the ATP synthase ...
... - energy used to pump hydrogen (H+) from matrix into intermembrane space - H+ becomes more concentrated (100 times) in intermembrane space as in matrix - H+ concentration gradient drives chemiosmosis - chemiosmosis is process of producing ATP from ADP + P by an enzyme complex called the ATP synthase ...
Biology 1406 Exam 2
... - energy used to pump hydrogen (H+) from matrix into intermembrane space - H+ becomes more concentrated (100 times) in intermembrane space as in matrix - H+ concentration gradient drives chemiosmosis - chemiosmosis is process of producing ATP from ADP + P by an enzyme complex called the ATP synthase ...
... - energy used to pump hydrogen (H+) from matrix into intermembrane space - H+ becomes more concentrated (100 times) in intermembrane space as in matrix - H+ concentration gradient drives chemiosmosis - chemiosmosis is process of producing ATP from ADP + P by an enzyme complex called the ATP synthase ...
Further Details of Mechanism
... • Hydrophobic tail of each is composed of 6 to 10 five-carbon isoprenoid units • The isoprenoid chain allows these quinones to dissolve in lipid membranes ...
... • Hydrophobic tail of each is composed of 6 to 10 five-carbon isoprenoid units • The isoprenoid chain allows these quinones to dissolve in lipid membranes ...
Chapter Outline
... 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H + to become water. 7. Glucose is oxidized and O2 is reduced. 8. The re ...
... 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H + to become water. 7. Glucose is oxidized and O2 is reduced. 8. The re ...
Energy - Doctor Jade Main
... ATP synthases built into the inner mitochondrial membrane make ATP as hydrogen ions are driven back across the membrane by the energy of the concentration gradient. Since the membrane is not permeable to hydrogen ions they can only cross via a channel in the membrane. This channel is through the ATP ...
... ATP synthases built into the inner mitochondrial membrane make ATP as hydrogen ions are driven back across the membrane by the energy of the concentration gradient. Since the membrane is not permeable to hydrogen ions they can only cross via a channel in the membrane. This channel is through the ATP ...
Biology 7th hour Chapter 6 Krebs Cycle and Fermentation Quiz
... a. It involves 9 distinct reactions c. It yields 2 molecules of CO2 b. It regenerates energy-rich NADH d. It produces a final end product _____ 6) The final electron acceptor in the process of respiration is: a) CO2 c) H2O b)Oxygen d) ATP _____ 7) During the Krebs cycle, the carbon atoms in glucose ...
... a. It involves 9 distinct reactions c. It yields 2 molecules of CO2 b. It regenerates energy-rich NADH d. It produces a final end product _____ 6) The final electron acceptor in the process of respiration is: a) CO2 c) H2O b)Oxygen d) ATP _____ 7) During the Krebs cycle, the carbon atoms in glucose ...
Chapter 9: Cellular Respiration and Fermentation
... c. The carbons have been lost in the molecule d. How many FADH2 have been formed? e. How many ATPs are formed? f. How many times does the citric acid cycle occur for each molecule of glucose? ...
... c. The carbons have been lost in the molecule d. How many FADH2 have been formed? e. How many ATPs are formed? f. How many times does the citric acid cycle occur for each molecule of glucose? ...
File - Hope Christian College Parent and Student Portal
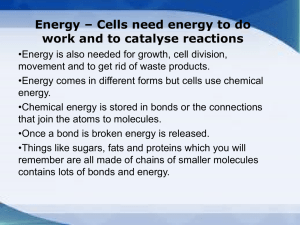
... Energy – Cells need energy to do work and to catalyse reactions •Energy is also needed for growth, cell division, movement and to get rid of waste products. •Energy comes in different forms but cells use chemical energy. •Chemical energy is stored in bonds or the connections that join the atoms to m ...
... Energy – Cells need energy to do work and to catalyse reactions •Energy is also needed for growth, cell division, movement and to get rid of waste products. •Energy comes in different forms but cells use chemical energy. •Chemical energy is stored in bonds or the connections that join the atoms to m ...
Chapter 4 Study Guide
... ______________ is broken down before the Krebs cycle. CO2 is released, NADH is produced, coenzyme a bonds to two molecules For every turn of the cycle, _______ molecule of ATP and _______ molecules of carbon dioxide are produced The Kreb cycle transports electrons to the _____________________ Kreb C ...
... ______________ is broken down before the Krebs cycle. CO2 is released, NADH is produced, coenzyme a bonds to two molecules For every turn of the cycle, _______ molecule of ATP and _______ molecules of carbon dioxide are produced The Kreb cycle transports electrons to the _____________________ Kreb C ...
Guided Reading Activities
... may have more than one characteristic or share characteristics. For glycolysis, put A; for citric acid cycle, put B; and for oxidative phosphorylation, put C. Occurs in the cytosol: ____________ Occurs in the mitochondria: ____________ Involves the splitting of glucose: ____________ Produces molecul ...
... may have more than one characteristic or share characteristics. For glycolysis, put A; for citric acid cycle, put B; and for oxidative phosphorylation, put C. Occurs in the cytosol: ____________ Occurs in the mitochondria: ____________ Involves the splitting of glucose: ____________ Produces molecul ...
File
... http://www.chimicare.org/curiosita/wpcontent/uploads/2011/11/schemasemplificato-fotosintesi-in-un-cloroplasto.gif ...
... http://www.chimicare.org/curiosita/wpcontent/uploads/2011/11/schemasemplificato-fotosintesi-in-un-cloroplasto.gif ...
Name
... 12) What kind of amino acids predominate in the region of a protein that passes through the plasma membrane? a) Acidic b) Basic c) Polar d) Non-polar 13) a) True b) False: All facilitated transport proteins require ATP hydrolysis to ADP + Pi in order for them to move materials across a membrane. 14) ...
... 12) What kind of amino acids predominate in the region of a protein that passes through the plasma membrane? a) Acidic b) Basic c) Polar d) Non-polar 13) a) True b) False: All facilitated transport proteins require ATP hydrolysis to ADP + Pi in order for them to move materials across a membrane. 14) ...
3.7 Energy-Rich Compounds
... energies of hydrolysis greater than - 30 kJ. By contrast, AMP is not energy-rich because its free energy of hydrolysis is only about half that of ADP or ATP (Figure 3.12). Although the energy released in ATP hydrolysis is -32 kJ, a caveat must be introduced here to define more precisely the energy r ...
... energies of hydrolysis greater than - 30 kJ. By contrast, AMP is not energy-rich because its free energy of hydrolysis is only about half that of ADP or ATP (Figure 3.12). Although the energy released in ATP hydrolysis is -32 kJ, a caveat must be introduced here to define more precisely the energy r ...
Photosynthesis: dark reactions
... and used to make amino acids • G-3-P (glyceraldehyde 3-P) is used to make fructose with is in turn used to make other sugars and starch • some fructose is converted into glucose; molecular of glucose are smaller and store more energy than ATP • fructose and glucose are used to make sucrose which is ...
... and used to make amino acids • G-3-P (glyceraldehyde 3-P) is used to make fructose with is in turn used to make other sugars and starch • some fructose is converted into glucose; molecular of glucose are smaller and store more energy than ATP • fructose and glucose are used to make sucrose which is ...
Plants
... 4. reaction center donates e- to electron transport chain (ETC) a. ETC is a series of redox rx b. stairs analogy 5. The ETC contains a proton pump a. pumps H+ into the thylakoid … b. [H+] increases and builds up pressure ...
... 4. reaction center donates e- to electron transport chain (ETC) a. ETC is a series of redox rx b. stairs analogy 5. The ETC contains a proton pump a. pumps H+ into the thylakoid … b. [H+] increases and builds up pressure ...
Plants
... 4. reaction center donates e- to electron transport chain (ETC) a. ETC is a series of redox rx b. stairs analogy 5. The ETC contains a proton pump a. pumps H+ into the thylakoid … b. [H+] increases and builds up pressure ...
... 4. reaction center donates e- to electron transport chain (ETC) a. ETC is a series of redox rx b. stairs analogy 5. The ETC contains a proton pump a. pumps H+ into the thylakoid … b. [H+] increases and builds up pressure ...
Advanced Biology
... Instructions: Read Chapter 8 in the Campbell text (chapter 6 if you still have the green book). Some questions may require you to look elsewhere in the textbook, or to make your own predictions or educated guesses. Please answer the questions thoroughly and in complete sentences. I encourage you to ...
... Instructions: Read Chapter 8 in the Campbell text (chapter 6 if you still have the green book). Some questions may require you to look elsewhere in the textbook, or to make your own predictions or educated guesses. Please answer the questions thoroughly and in complete sentences. I encourage you to ...
BI211StudyObjectivesChapters6
... 10. Indicate where each stage of aerobic respiration takes place in a eukaryotic cell. 11. Add up the energy captured (as ATP, NADH, and FADH2) in each stage of aerobic respiration. 12. Understand the electron transport chain, and define chemiosmosis, and explain how a gradient of protons is establi ...
... 10. Indicate where each stage of aerobic respiration takes place in a eukaryotic cell. 11. Add up the energy captured (as ATP, NADH, and FADH2) in each stage of aerobic respiration. 12. Understand the electron transport chain, and define chemiosmosis, and explain how a gradient of protons is establi ...
chapter review questions
... The cholesterol transported by HDLs is destined for destruction. HDLs transport cholesterol to the peripheral tissues for biosynthesis of steroid hormones. HDLs transport cholesterol to adipose tissue. ...
... The cholesterol transported by HDLs is destined for destruction. HDLs transport cholesterol to the peripheral tissues for biosynthesis of steroid hormones. HDLs transport cholesterol to adipose tissue. ...
ATP - mrs-shore
... What is the significance? It is universal with all living things Easily participates in many reactions Drives most biological processes One molecule can be synthesised and perform a large number of jobs • Only one system is used to deliver to many reactions ...
... What is the significance? It is universal with all living things Easily participates in many reactions Drives most biological processes One molecule can be synthesised and perform a large number of jobs • Only one system is used to deliver to many reactions ...
Diagram Sodium has 11 protons and 11 neutrons in its nucleus
... D. van der Waals forces 6. Which process changes a chlorine atom into a chloride ion? H: Gains a negative charge with this particle A. electron gain C. proton gain B. electron loss D. proton loss 7. Which of the following is a pure substance that cannot be broken down by a chemical reaction? H: its ...
... D. van der Waals forces 6. Which process changes a chlorine atom into a chloride ion? H: Gains a negative charge with this particle A. electron gain C. proton gain B. electron loss D. proton loss 7. Which of the following is a pure substance that cannot be broken down by a chemical reaction? H: its ...
313EnergyProduction
... – FFA in blood enter fibers by diffusion • rate of entry regulated by it’s own concentration gradient • increased FFA in blood drives FFA into muscle ...
... – FFA in blood enter fibers by diffusion • rate of entry regulated by it’s own concentration gradient • increased FFA in blood drives FFA into muscle ...
4.3 Photosynthesis in Detail
... membranes that aid in converting ADP to ATP by transferring electrons. • ATP synthase – Enzyme that catalyzes the reaction that adds a high-energy phosphate group to ADP to form ATP. • Calvin Cycle – Process by which a photosynthetic organism uses energy to synthesize simple sugars from CO2. ...
... membranes that aid in converting ADP to ATP by transferring electrons. • ATP synthase – Enzyme that catalyzes the reaction that adds a high-energy phosphate group to ADP to form ATP. • Calvin Cycle – Process by which a photosynthetic organism uses energy to synthesize simple sugars from CO2. ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.