- Free Documents
... Transport Chain consists of four separate respiratory complexes. rather a cyclic flow of electrons involves CoQ twice. The overall reaction is CoQH cyt cFeIII CoQ cyt cFeII H The flow of electrons from CoQH to the other components of the complex does not take a simple direct path. CoQHcytochrome c o ...
... Transport Chain consists of four separate respiratory complexes. rather a cyclic flow of electrons involves CoQ twice. The overall reaction is CoQH cyt cFeIII CoQ cyt cFeII H The flow of electrons from CoQH to the other components of the complex does not take a simple direct path. CoQHcytochrome c o ...
Chapter 7
... Stage 4: Oxidative phosphorylation High energy electrons removed from NADH and FADH2 to make ATP Typically requires oxygen Oxidative process involves electron transport chain Phosphorylation occurs by ATP synthase ...
... Stage 4: Oxidative phosphorylation High energy electrons removed from NADH and FADH2 to make ATP Typically requires oxygen Oxidative process involves electron transport chain Phosphorylation occurs by ATP synthase ...
Unit 3: Energy systems
... pyruvate molecules created from glycolysis. When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur, leading to _______________. In the presence of oxygen, when acety ...
... pyruvate molecules created from glycolysis. When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur, leading to _______________. In the presence of oxygen, when acety ...
Fibre types
... Aerobically in the mitochondria = 36 ATP or Anaerobically in the cytoplasm = 1or 2ATP 1 molecule of PC gives 1 molecule of ATP 1 molecule of glucose gives 2 ATP + Lactate ...
... Aerobically in the mitochondria = 36 ATP or Anaerobically in the cytoplasm = 1or 2ATP 1 molecule of PC gives 1 molecule of ATP 1 molecule of glucose gives 2 ATP + Lactate ...
CHAPTER 8 CELLULAR RESPIRATION
... 1. Cellular respiration includes four phases: a. Glycolysis is the breakdown of glucose in the cytoplasm into two molecules of pyruvate. 1) Enough energy is released for immediate buildup of two ATP. 2) Glycolysis takes place outside the mitochondria and does not utilize oxygen. b. In the transition ...
... 1. Cellular respiration includes four phases: a. Glycolysis is the breakdown of glucose in the cytoplasm into two molecules of pyruvate. 1) Enough energy is released for immediate buildup of two ATP. 2) Glycolysis takes place outside the mitochondria and does not utilize oxygen. b. In the transition ...
THE CELLULAR RESPIRATION SAGA II: THE CITRIC ACID CYCLE
... • Step 2: Oxidize 6 C molecule to give NADH • CO2 is released • Left with 5 C molecule ...
... • Step 2: Oxidize 6 C molecule to give NADH • CO2 is released • Left with 5 C molecule ...
Photosynthesis & Cellular Respiration
... • Photosynthesis- is the process that converts the radiant energy of sunlight into chemical energy (glucose) • Respiration-the process that releases chemical energy for use by the cell (in the form of ATP) ...
... • Photosynthesis- is the process that converts the radiant energy of sunlight into chemical energy (glucose) • Respiration-the process that releases chemical energy for use by the cell (in the form of ATP) ...
bioc-2200-a-biol-2200-a-mock-final-exam
... 1. Choose the list of intermediates in the correct order for the citric acid cycle a. succinyl-CoA, succinate, a-ketoglutarate, fumarate, Malate b. α-ketoglutarate, succinyl-CoA, succinate, fumarate, Malate c. succinate, succinyl-CoA, fumarate, α-ketoglutarate, Malate d. α -ketoglutarate, succinyl-C ...
... 1. Choose the list of intermediates in the correct order for the citric acid cycle a. succinyl-CoA, succinate, a-ketoglutarate, fumarate, Malate b. α-ketoglutarate, succinyl-CoA, succinate, fumarate, Malate c. succinate, succinyl-CoA, fumarate, α-ketoglutarate, Malate d. α -ketoglutarate, succinyl-C ...
Metabolism08
... From Carbohydrate – glucose From Lipids – glycerol and fatty acids From Protein – amino acids ...
... From Carbohydrate – glucose From Lipids – glycerol and fatty acids From Protein – amino acids ...
BIOLOGY 311C - Brand Spring 2007 NAME (printed very legibly
... a. CO2 and ATP. b. sugar and NADH. c. ATP and NADPH. d. ADP and NADP+. 32. The CO2-fixing enzyme, rubisco, also uses a non-productive reactant, which causes photorespiration. This non-productive substrate is: a. NH4+ . b. N2 . c. HCO3 -. d. O2 . 33. CAM plants differ from normal C3 plants in that CA ...
... a. CO2 and ATP. b. sugar and NADH. c. ATP and NADPH. d. ADP and NADP+. 32. The CO2-fixing enzyme, rubisco, also uses a non-productive reactant, which causes photorespiration. This non-productive substrate is: a. NH4+ . b. N2 . c. HCO3 -. d. O2 . 33. CAM plants differ from normal C3 plants in that CA ...
Nucleotides: Be able to differentiate between a purine ring and a
... base (isoalloxazine – don’t need to know this) and the sugar ribitol (like ribose but the aldehyde is reduced to an alcohol) this is also a dinucleotide, and one of the nucleotides has the adenine base. the other nucleotide contains the nitrogenous base present in riboflavin. A related molecule is f ...
... base (isoalloxazine – don’t need to know this) and the sugar ribitol (like ribose but the aldehyde is reduced to an alcohol) this is also a dinucleotide, and one of the nucleotides has the adenine base. the other nucleotide contains the nitrogenous base present in riboflavin. A related molecule is f ...
WHAT SHOULD I KNOW ABOUT RESPIRATION NAME ANSWERS
... alcoholic fermentation – Takes place in cytoplasm without oxygen Uses energy from NADH to change pyruvic acid into alcohol and releases CO2 ; NAD+ is regenerated; lactic acid fermentation – Takes place in cytoplasm without oxygen Uses energy from NADH to change pyruvic acid into lactic acid; NAD + i ...
... alcoholic fermentation – Takes place in cytoplasm without oxygen Uses energy from NADH to change pyruvic acid into alcohol and releases CO2 ; NAD+ is regenerated; lactic acid fermentation – Takes place in cytoplasm without oxygen Uses energy from NADH to change pyruvic acid into lactic acid; NAD + i ...
Chapter 8 Microbial Metabolism
... Cell Respiration: Electron Transport System and the Proton Motive Force As glucose was oxidized you noticed that there was a fair amount of reducing power formed (NADH and FADH2). As NAD+ and FAD are reduced they carry the electrons to the cell membrane which is the site of the electron transport sy ...
... Cell Respiration: Electron Transport System and the Proton Motive Force As glucose was oxidized you noticed that there was a fair amount of reducing power formed (NADH and FADH2). As NAD+ and FAD are reduced they carry the electrons to the cell membrane which is the site of the electron transport sy ...
Nitrate (NO3) + (e
... a. Organism uses molecules other than O2 as final electron acceptor. b. Oxygen is toxic since it binds the electrons before ATP can be made e- ...
... a. Organism uses molecules other than O2 as final electron acceptor. b. Oxygen is toxic since it binds the electrons before ATP can be made e- ...
CellularRespirationglycolysis
... uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane – These ions store potential energy ...
... uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane – These ions store potential energy ...
Chapter 8 Enzymes: basic concepts and kinetics
... • Reducing equivalents are transferred to an electron transport chain, a respiratory chain. • Free energy is stored in a proton gradient that drives the synthesis of ATP. ...
... • Reducing equivalents are transferred to an electron transport chain, a respiratory chain. • Free energy is stored in a proton gradient that drives the synthesis of ATP. ...
031607
... – High specificity and efficiency relative to inorganic catalysts, for example – Participate in reactions, but no net change – Lower the activation energy – Do not change equilibrium (get there faster) ...
... – High specificity and efficiency relative to inorganic catalysts, for example – Participate in reactions, but no net change – Lower the activation energy – Do not change equilibrium (get there faster) ...
20121016083538
... making ATP energy (& some heat) by burning fuels in many small steps ATP Regents Biology ...
... making ATP energy (& some heat) by burning fuels in many small steps ATP Regents Biology ...
Cell Respiration
... All of the reactions of glucose oxidation that follow glycolysis involving the transfer of electrons to their final acceptor, oxygen, take place in eukaryotic cells in the ___________. ...
... All of the reactions of glucose oxidation that follow glycolysis involving the transfer of electrons to their final acceptor, oxygen, take place in eukaryotic cells in the ___________. ...
Exam 2
... catalyzes reactions as fast as the enzyme encounters substrate. 18. For an allosteric enzyme, the shape of its velocity vs. substrate concentration graph is _____________________________________. 19. When oxygen is bound to the heme group of hemoglobin, it is prevented from oxidizing the iron by the ...
... catalyzes reactions as fast as the enzyme encounters substrate. 18. For an allosteric enzyme, the shape of its velocity vs. substrate concentration graph is _____________________________________. 19. When oxygen is bound to the heme group of hemoglobin, it is prevented from oxidizing the iron by the ...
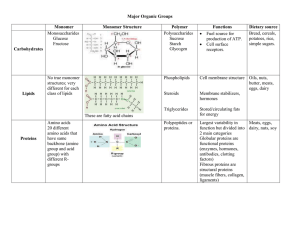
Major Organic Groups - Lemon Bay High School
... Largest variability in Meats, eggs, function but divided into dairy, nuts, soy 2 main categories Globular proteins are functional proteins (enzymes, hormones, antibodies, clotting factors) Fibrous proteins are structural proteins (muscle fibers, collagen, ligaments) ...
... Largest variability in Meats, eggs, function but divided into dairy, nuts, soy 2 main categories Globular proteins are functional proteins (enzymes, hormones, antibodies, clotting factors) Fibrous proteins are structural proteins (muscle fibers, collagen, ligaments) ...
Document
... The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules because each contains a pair of electrons having a high transfer potential. When these electrons are used to reduce molecular oxygen to water, a large amount of free energy is liberated ...
... The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules because each contains a pair of electrons having a high transfer potential. When these electrons are used to reduce molecular oxygen to water, a large amount of free energy is liberated ...
Ch. 9 - Crestwood Local Schools
... use these as energy sources as well! Proteins first broken down into AA’s Amino group (containing N) is removed from each AA by deamination Converts ...
... use these as energy sources as well! Proteins first broken down into AA’s Amino group (containing N) is removed from each AA by deamination Converts ...
Elements PPT
... small there is 10 to the 23rd atoms in a gram of water Contain positively charged protons, neutral neutrons, and negatively charged electrons Neutrons and protons make up the nucleus of an atom, while electrons orbit around the nucleus Each element has a unique arrangement of protons, neutrons, or e ...
... small there is 10 to the 23rd atoms in a gram of water Contain positively charged protons, neutral neutrons, and negatively charged electrons Neutrons and protons make up the nucleus of an atom, while electrons orbit around the nucleus Each element has a unique arrangement of protons, neutrons, or e ...
ATP
... Glucose loses electrons (in H atoms) and becomes oxidized O2 gains electrons (in H atoms) and becomes reduced Along the way, the electrons lose potential energy, and energy is released ...
... Glucose loses electrons (in H atoms) and becomes oxidized O2 gains electrons (in H atoms) and becomes reduced Along the way, the electrons lose potential energy, and energy is released ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.