* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Medial medullary syndrome
Survey
Document related concepts
Functional magnetic resonance imaging wikipedia , lookup
Metastability in the brain wikipedia , lookup
Brain morphometry wikipedia , lookup
Synaptic gating wikipedia , lookup
Perivascular space wikipedia , lookup
Diffusion MRI wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Hypothalamus wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Aging brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Transcript
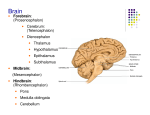
Pathology Spectrum of the Medulla Oblongata: A Neuroimaging Review Jason Kramer MD Kathleen Barry MD, FACR Ay-Ming Wang MD, FACR Anant Krishnan, MD Jeffrey Wilseck, DO, FAOCR Division of Neuroradiology Department of Diagnostic Radiology and Molecular Imaging Beaumont Health System Oakland University William Beaumont School of Medicine eEdE-11 • Anatomy of the medulla oblongata Cerebrovascular accident Hypoxic ischemic encephalopathy Cavernous malformation Dural arteriovenous fistula Traumatic brain injury Metastasis Ependymoma Glioblastoma multiforme Multiple sclerosis Hypertrophic olivary degeneration Multisystem atrophy Wallerian degeneration Viral encephalitis Wernicke encephalopathy Leigh disease Chiari malformation Conclusion • The medulla oblongata is the lowest portion of the brainstem and continuous with the pons superiorly and spinal cord inferiorly. It is a vital anatomic structure as it is responsible for multiple autonomic functions necessary for life. It contains the cardiac, respiratory, vomiting, and vasomotor centers, therefore the medulla oblongata is crucial for breathing, heart rate, and blood pressure. Neurons of the reticular formation play a central role in transmission of motor and sensory impulses and controls such functions as arousal and sleep. The medulla oblongata also contains multiple reflex centers responsible for vomiting, coughing, sneezing, and swallowing. • The medulla oblongata may be anatomically divided into two main parts: the ventral medulla and dorsal medulla (tegmentum). • The ventral medulla contains the pyramids, within which course the pyramidal tracts made up of the corticospinal tract and corticobulbar tract, as well as the olivary bodies located laterally. • The dorsal medulla forms the inferior fourth ventricle at its upper aspect and contains the brainstem nuclei for cranial nerves IX-XII. Pyramidal tracts Corticobulbar tract: Two neuron white matter motor pathway connecting the cerebral cortex to the brainstems, primarily involved in carrying the motor function of the nonoculomotor cranial nerves. Corticospinal tract: Lateral and anterior components. Lateral corticospinal tract controls fine movement of limbs. Anterior corticospinal tract also involves fine motor control of the limbs as well as more central axial and girdle musculature. Nucleus solitarius: Receives taste sensation, sensory information from the ear, chemoreceptors and mechanoreceptors of the general visceral afferent pathway including the carotid and aortic bodies, heart, lungs, airways, GI tract, pharynx, and liver. Mediates the gag reflex, carotid sinus reflex, aortic reflex, cough reflex, baroreceptor and chemoreceptor reflexes, several respiratory reflexes, and reflexes mediating GI motility and secretion. Spinal trigeminal nucleus: Receives information about deep touch, pain, and temperature from the ipsilateral face. Receives input from cranial nerves V, VII, IX, and X. Inferior olivary nucleus: Closely associated with the cerebellum. Involved in control and coordination of movements, sensory processing and cognitive tasks Hypertrophy has been associated with progressive supranuclear palsy. Nucleus ambiguus: Controls motor innervation of ipsilateral muscles of the soft palate, pharynx, larynx, and upper esophagus. Preganglionic parasympathetics to the heart also course through the external formation of the nucleus. Medial lemniscus: Formed by the crossings of the internal arcuate fibers that compose the nucleus gracilis and nucleus cuneatus. Part of the posterior column-medial lemniscus pathway which ascends from the skin to the thalamus. Important for vibration and touch-pressure sensation from the skin and joints. Nucleus cuneatus: Part of the posterior column-medial lemniscus pathway. Carries fine touch and proprioception information from the upper body to the contralateral thalamus via the medial lemniscus. Nucleus gracilis: Contains second-order neurons of the posterior column-medial lemniscus pathway. Participates in fine touch and proprioception sensation of the lower body (legs and trunk). a. b. c. (a., b.) Diffusion weighted imaging and ADC map demonstrates diffusion restriction in the left medulla oblongata consistent with acute/early subacute ischemia. (c.) Coronal MRA 3D MIP image demonstrates critical stenosis of the left vertebral artery V4 segment. • The arterial supply of the medulla oblongata is via: • Posterior inferior cerebellar artery (PICA): Supplies the lateral medulla oblongata • Anterior spinal artery: Supplies the medial medulla oblongata • Direct branches arising from the vertebral artery and proximal basilar artery: Supply an area between the other two main arteries. • Ischemia to different vascular distributions produce different characteristic neurologic deficits. • Lateral medullary syndrome (Wallenberg syndrome): Acute ischemic infarct of the lateral medulla oblongata, most commonly due to occlusion of the intracranial vertebral artery or PICA, characterized by: • Vestibulocerebellar symptoms: Falling towards the side of the lesion, diplopia, and multidirectional nystagmus (inferior cerebellar peduncle and vestibular nucleus) • Autonomic dysfunction: Ipsilateral Horner’s syndrome, hiccups • Sensory symptoms: Initial sharp pain at the ipsilateral face followed by contralateral loss of pain and temperature sensation (spinal trigeminal nucleus involvement) • Ipsilateral bulbar muscle weakness: Hoarseness, dysphonia, dysphagia, dysarthria, decreased gag reflex (nucleus ambiguus) • Medial medullary syndrome (Dejerine syndrome): Rare entity typically caused by thrombotic or embolic occlusion of anterior spinal artery, small perforating branches arising from the vertebral or proximal basilar artery supplying the medial aspect of the medulla oblongata characterized by: • Contralateral hemiplegia/hemiparesis of trunk and extremities • Contralateral loss of proprioception, discriminative tactile sensation, and vibration sensation from the trunk and extremities • Ipsilateral hypoglossal palsy (tongue weakness, fasciculations, and atrophy, due to involvement of the hypoglossal nucleus) • Vertigo • Nausea • Ipsilateral limb ataxia Diffusion weighted imaging demonstrates bilateral symmetric diffusion restriction in the medulla oblongata, pons, midbrain, medial temporal lobes and hippocampi, thalami, basal ganglia, mammillary bodies, and cerebral cortex. • Potentially devastating neurologic injury developing in varying regions in the brain depending on severity and duration of hypoperfusion and oxygenation. • Particular sites with high energy demands are most vulnerable to cytotoxic crisis in the event of hypoxia/hypoxemia. • Usual neuroanatomical locations include the cerebral cortex, cerebellum, hippocampus, basal ganglia, and thalamus. More severe cases may involve the entire limbic system, cerebral white matter, and brainstem. • Most conspicuous neuroimaging findings include diffusion restriction at the sites of injury on DWI. Subtle FLAIR hyperintensity may be detectable. In more severe cases, global cerebral edema may occur. • White matter involvement may be overlooked on conventional T2WI because markedly edematous grey matter may make the white matter appear to have relatively low signal intensity and therefore appear normal. DWI greatly facilitates avoiding this pitfall. a. d. c. b. e. f. g. h. (a-c) Axial CT images without contrast demonstrate small parenchymal hemorrhages in the dorsal medulla oblongata, left pons, and left lentiform nucleus secondary to multiple underlying cavernous malformations. (d-f) Axial gradient echo T2* images demonstrate susceptibility artifact in the dorsal medulla, left pons, and left lentiform nucleus due to hemosiderin. (g,h) Axial T1W1 with contrast images demonstrate heterogeneous signal consistent with blood products of different ages. There is no enhancement. • Slow flow vascular malformation defined in histologic terms by blood cavities surrounded by a single layer of endothelium without muscular tissue or intervening brain parenchyma. • Often asymptomatic, although may present with seizures, hemorrhage, focal neurologic signs, and headache. • MRI is most sensitive modality for diagnosis. There is classically susceptibility artifact on gradient recalled echo sequence as well as a peripheral halo of T2 shortening due to the presence of hemosiderin. These lesions do not typically enhance and are angiographically occult. • Most commonly occur sporadically as a solitary lesion, although rarely may occur as an autosomal dominant familial form with multiple lesions (most often described in the Hispanic American population.) • Most commonly supratentorial (80-85%), although may occur throughout the CNS. • Present with hemorrhage at time of diagnosis in approximately 15% of cases. • Commonly associated with developmental venous anomalies. a. b. c. d. (a.) Axial T2WI and (b.) FLAIR images demonstrate hyperintense signal within the medulla oblongata secondary to venous congestion and associated vasogenic edema. (c.) Axial contrast-enhanced T1WI demonstrates prominent vessels adjacent to the medulla oblongata (arrow) relating to a dural arteriovenous fistula. (d.) Digital subtraction angiography in the lateral projection with transcatheter injection of the right ascending pharyngeal artery branch of the external carotid artery (yellow arrow) demonstrates early venous filling at the site of the dural arteriovenous fistula (blue arrow) and multiple prominent venous structures coursing along the brainstem and medulla oblongata (red arrows). This was a confirmed dural arteriovenous fistula with arterial supply from the neuromeningeal branches of the ascending pharyngeal artery. CBF MRI perfusion demonstrates decreased cerebral blood flow (CBF) and cerebral blood volume (CBV) at the medulla oblongata with increased time-to-peak (TTP). These findings relate to decreased perfusion from steal phenomenon caused by the dural arteriovenous fistula. TTP CBV • Fistulous communication between arteries that would normally feed the meninges, bone, or muscles, but not the brain, and small venules within the dura matter. • Account for 10-15% of all intracranial arteriovenous shunts. • Several classification systems have been described, with perhaps the simplest method being to group lesions into those with and those without cortical venous reflux. • Lesions without cortical venous reflux (Borden type 1) almost never lead to neurologic deficits. • Lesions with cortical venous reflux (Borden types 2 and 3) often have an aggressive clinical course including intracranial hemorrhage, seizure, dementia, altered consciousness, and focal neurologic symptoms due to venous congestion or rupture. Dural AVF with cortical venous reflux will manifest with abnormal vessels present outside the brain parenchyma. • Neuroimaging findings include dilated cortical veins which manifest as abnormal enhancing tubular structures or flow voids within the cortical sulci with no true nidus within the brain parenchyma. • MRA, CTA, and DSA will demonstrate early venous filling with arterial supply from external carotid artery branches. a. b. c. d. (a.) Axial noncontrast CT images demonstrate parenchymal hematomas in the right frontal and occipital lobes and subarachnoid hemorrhage within the left Sylvian fissure. (b., c.) Axial FLAIR and (d.) T2WI demonstrates hyperintense signal within the right ventral medulla oblongata presumably related to traumatic contusion. The patient was status post motor vehicle accident and was comatose upon presentation. • CT is the first imaging test performed in the emergency setting for evaluation of head trauma, the goal being to identify lesions requiring emergent neurosurgical treatment. • CT is important in the emergency setting of traumatic brain injury due to its fast acquisition time and widespread availability. • MRI is reserved for evaluation when CT does not fully explain patient symptoms or to better delineate CT findings. MRI is being increasingly used in the emergency setting for these reasons. • MRI has much higher sensitivity for detecting microhemorrhages and diffuse axonal injury, especially when protocol includes a T2* gradient recall echo sequence or susceptibility weighted imaging (SWI). a. b. c. d. (a.) Axial T1WI with contrast demonstrates a peripherally enhancing intra-axial mass in the right frontal lobe with surrounding vasogenic edema consistent with a large metastatic focus. (b.) Coronal T1WI with contrast demonstrates a peripherally enhancing mass in the left upper medulla oblongata consistent with a metastatic lesion. (c, d.) Axial and sagittal T1WI with contrast demonstrates the peripherally enhancing metastatic lesion in the posterior upper medulla oblongata. (e.) Axial chest CT demonstrates a spiculated central mass in the right upper lobe proven to be a primary squamous cell carcinoma of the lung. e. • Neoplasms with tendency to metastasize to the brain, spinal cord, or meninges may also metastasize to the medulla oblongata. • Metastatic disease to the medulla oblongata may cause dysfunction of vital medullary nuclei and lower cranial nerve palsies either by direct infiltration or by associated vasogenic edema . • Cases of fatal autonomic dysfunction have been described in the literature attributed to metastatic disease to the medulla oblongata. a. b. c. d. e. (a.) Sagittal T2WI image demonstrates an expansile intrinsic mass in the posterior medulla oblongata with a large cystic component. (b-e) Sagittal, coronal, and axial contrast-enhanced T1WI demonstrate the expansile intrinsic mass in the posterior medulla oblongata. The mass is predominantly cystic, but demonstrates an enhancing solid component at its anterior aspect suggestive of neoplasm. This mass was surgically resected and pathologically proven to be an ependymoma. • WHO grade II or III glial neoplasm arising from ependymal cells with a variety of subtypes. • May occur anywhere along the neuraxis, therefore imaging the entire neuraxis is important prior to treatment. • Most commonly located in the posterior fossa, classically arising from the floor of the 4th ventricle and often protruding out the foramen of Luschka (“plastic tumor”). • Demonstrates heterogeneous neuroimaging characteristics due to propensity to hemorrhage and contain calcifications. • May appear as a solid tumor or cystic with solid components. • When tumor involves the medulla oblongata, complete surgical resection is often difficult due to infiltrating nature of the tumor and adherence to adjacent structures vital to autonomic functions necessary for life. a. b. c. (a.) Axial contrast-enhanced T1WI demonstrates a large intra-axial mass in the right parieto-occipital lobe with irregular peripheral enhancement and central necrosis. There is significant mass effect with effacement of the right lateral ventricle and leftward subfalcine herniation. (b.) Sagittal contrastenhanced T1WI demonstrates a smaller enhancing mass at the dorsal caudal medulla oblongata. (c.) Axial contrast-enhanced T1WI demonstrates the smaller enhancing mass at the left dorsal medulla oblongata. This was pathologically proven to represent multicentric glioblastoma multiforme. • GBM is the most common glial tumor of the adult brain, however primary GBM of the medulla oblongata is rare. • Neuroimaging features of GBM of the medulla oblongata are consistent with those of GBM at other sites, often appearing as a heterogeneous mass with ring-like enhancement, a poorly defined margin, and tumoral hemorrhage. When located in the caudal brainstem, it may demonstrate an exophytic nature. Although rare, GBM should be included in the differential diagnosis of tumors of the caudal brainstem. • WHO grade IV tumor with poor prognosis, median survival approximately 2 years. • Complete surgical resection may prolong survival somewhat, although may severely impair quality of life due to infiltrative and adherent nature of the tumor. Total surgical resection may have dire consequences on autonomic functions of the medulla oblongata with associated dysfunction of the lower cranial nerves, and therefore subtotal resection is a considered treatment option. However, with subtotal resection, tumor progression will inevitably impair function as well. a. b. c. d. (a.) Axial FLAIR image demonstrates focal hyperintense signal in the right lateral medulla oblongata relating to a demyelinating lesion in the setting of multiple sclerosis. (b.) Axial T1WI demonstrates corresponding hypointense signal at the demyelinating lesion. Presence of hypointense signal on T1WI has been associated with worse prognosis. (c, top image.) Sagittal T2 FLAIR demonstrates multifocal signal hyperintensities in the periventricular white matter. The lesions are oriented perpendicular to the callososeptal interface in a perivenular distribution along the path of the deep medullary veins, which is characteristic of the demyelinating lesions in the setting of multiple sclerosis. These lesions are commonly referred to as “Dawson’s fingers”. (c, bottom image) Sagittal T2 FLAIR demonstrates the focal demyelinating lesion in the upper medulla oblongata. (d.) Axial FLAIR image demonstrates multiple demyelinating lesions in the bilateral periventricular white matter. • MS is the most common chronic inflammatory demyelinating disease affecting the central nervous system. • MRI criteria for diagnosis of MS in patients with clinical syndrome suggestive of MS include demonstration of disease dissemination in time and space. • Active lesions may demonstrate diffusion restriction and/or enhancement. • Lesions that appear hypointense on T1WI, aka “black holes”, are associated with more severe tissue damage. • Characteristic neuroimaging findings include demyelinating lesions in the periventricular white matter which are classically oriented perpendicular to the corpus callosum in a perivenular distribution along the path of the deep medullary veins (“Dawson’s fingers”). • Enhancement of the optic nerves is also a characteristic finding in the presence of optic neuritis. • Brainstem and spinal cord involvement is common. Brainstem lesions may result in cranial neuropathies and even autonomic dysfunction. • MS involvement of the medulla oblongata has rarely been associated with sudden cardiac death. Sudden arrhythmias have occurred with MS lesions affecting the medulla oblongata due to dysfunction of the cardiac autonomic innervation which the medulla oblongata controls. a. b. c. (a.) Sagittal T1WI, (b.) axial T2WI, and (c.) axial FLAIR image demonstrates intraparenchymal hemorrhage within the caudal midbrain and posterior pons in a patient who presented with hemorrhagic stroke and later developed bilateral hypertrophic olivary degeneration (next slide). a. b. c. Follow up imaging 2 years later. (a.) Axial T2WI, (b.) FLAIR, and (c.) T1WI demonstrates prominence of the medulla oblongata inferior olivary nuclei with associated T1 and T2 prolongation related to demyelination and vacuolization. Note the bilateral nature of the lesions. Hypertrophic olivary degeneration is most commonly a unilateral process, however can be bilateral if caused by a lesion involving the midbrain corresponding to the region of the Werneking decussation (decussation of the superior cerebellar peduncle or brachium conjunctivum), as in our patient. • Trans-synaptic degeneration with neuronal loss and reactive gliosis in the medullary olives after losing synaptic input from injury to their afferent fibers. • HOD is a unique form of degeneration because it results in enlargement of the affected structure rather than atrophy. • Follows injury to the dentato-rubral-olivary pathway, or “triangle of Guillain and Mollaret”, which is the afferent pathway to the medullary olive comprised of connections between the inferior olivary nucleus, red nucleus, and contralateral dentate nucleus. • Onset is typically preceded by a pontine hemorrhage involving the ipsilateral central tegmental tract, contralateral superior cerebellar peduncle, or dentate nucleus, although may be preceded by any type of injury to these structures. • Clinical symptoms include palatal myoclonus and uncontrollable tremor, presumably caused by loss of inhibitory control. • Neuroimaging findings include a non-enhancing T1 isointense, T2 hyperintense enlargement confined to the olivary nucleus related to demyelination, vacuolization, and cell death of neurons and astrocytes. The findings develop around 6 months after then event and resolve after 3-4 years, however clinical symptoms persist. (a.) Sagittal T1WI demonstrates marked atrophy of the brainstem with flattening of the anterior pons and medulla oblongata. (b.) Axial FLAIR image demonstrates atrophy of the medulla oblongata. Note the atrophy of the cerebellar vermis. (c.) Axial FLAIR demonstrates marked atrophy of the pons and middle cerebellar peduncles with subtle hyperintense signal abnormality. (d.) Axial FLAIR demonstrates atrophy of the midbrain with associated abnormal hyperintense signal. (e.) Axial T2WI demonstrates cruciform signal abnormality within the pons. This is the “hot cross bun sign”. (f). Axial T2WI demonstrates marked hypointense signal in the bilateral putamina secondary to iron deposition. Note the subtle hyperintense signal along the lateral putaminal margins. This is the “putaminal rim sign”. a. b. d. e. c. f. • Neurodegenerative disorder clinically resembling Parkinson’s disease with neuronal loss and gliosis in the inferior olives, pons, cerebellum, substantia nigra, locus ceruleus, striatum, and intermediolateral column of the spinal cord. • May be grouped into parkinsonian type in which substantia nigra is the main site affected and cerebellar ataxia type in which the olivopontocerebellar system is mainly involved. • Neuroimaging findings include olivopontocerebellar atrophy, putaminal atrophy, and abnormal T2 prolongation within the pons and middle cerebellar peduncles thought to relate to degeneration of pontocerebellar fibers. This signal abnormality may be cruciform (“hot cross bun sign”). Additional findings include hypointensity in the putamina on T2* and T2WI related to iron deposition, and a thin rim of T2 prolongation along the posterolateral putamina related to reactive microgliosis and astrogliosis (“putaminal rim sign”). Axial T2WI demonstrate volume loss within the left cerebral peduncle and left medulla oblongata secondary to Wallerian degeneration in an 11 month old patient with remote left middle cerebral artery territory infarction. There is cystic encephalomalacia seen in the ipsilateral temporal lobe. • Process of axonal degeneration distal to injury of the neuronal cell body or proximal axon which may begin within 1 week of damage and continue during next 6 months. Signifies irreversible loss of neuronal function. • Most common cause is cerebral infarction, but may also result from hemorrhage, neoplasm, trauma, white matter disorders, and multisystem atrophy. • Most frequently observed in the corticospinal tract following infarction of the motor cortex or internal capsule. • Has also been described in the optic radiations, corpus callosum, spinal cord, and limbic system. • Presence or absence of Wallerian degeneration may influence clinical outcome after stroke. • Neuroimaging findings are time-dependent and best detected on MRI. Initially will appear T1 hyperintense and T2 hypointense, then T1 hypointense and T2 hyperintense, and finally with ipsilateral brainstem atrophy sometimes with persistent signal abnormality. • Diffusion weighted imaging may detect Wallerian degeneration in its earliest stage before T1/T2 signal abnormalities present. • Diffusion tensor imaging may distinguish between a primary lesion and associated Wallerian degeneration. Diffusion weighted images (above left) demonstrate hyperintense signal within the dorsal medulla oblongata, central tegmental tract, cerebellar vermis, midbrain periaqueductal gray matter, hippocampi, and medial thalami in a bilateral symmetric distribution. There was corresponding hypointense signal on ADC map consistent with diffusion restriction. There was also hyperintense signal on T2WI/FLAIR at the sites of abnormality, as demonstrated on the FLAIR images above right. Follow up imaging was performed 5 days later after treatment. The FLAIR (top) and diffusion (bottom) signal abnormalities had resolved. • Severe neurologic complication of influenza, typically affecting young children, characterized by an abrupt onset of seizures and coma within a few days of developing a high fever. • Most feared complications are acute necrotizing encephalopathy, Reye’s syndrome (rapidly progressive postviral encephalopathy), and hemorrhagic shock. • Adult onset is associated with increased incidence of stroke and myocardial infarction. • Mortality rate is as high as 30% without treatment. • Neuroimaging findings are variable and may include symmetric T2 prolongation involving the supratentorial white matter, thalamus, splenium, brain stem, and/or cerebellum, often associated with diffusion restriction. These findings are thought to relate to an influx of inflammatory cells and macromolecules causing cytotoxic edema. • Anti-influenza treatments such as a selective neuraminidase inhibitor (oseltamivir phosphate), corticosteroids, and hypothermia are quite effective. • Clinical improvement may precede radiologic improvement. TOP: Diffusion weighted imaging demonstrate symmetric diffusion restriction in the bilateral dorsal medulla oblongata, facial colliculi, periaqueductal gray matter, and medial thalami. BOTTOM: Axial FLAIR images demonstrate hyperintense signal intensity at the sites of diffusion restriction. • Acute neurological syndrome resulting from thiamine (vitamin B1) deficiency. • Clinical conditions associated with impaired thiamine absorption include chronic alcohol abuse, gastrointestinal surgery, prolonged vomiting, chemotherapy, systemic infectious and noninfectious disease, and dietary unbalance. • Classical clinical triad of confusion, ataxia, and ophthalmoplegia is actually seen in a minority of patients (16-38%). • Ocular sings include nystagmus, bilateral lateral rectus palsies, and conjugate gaze palsies reflecting involvement of the oculomotor, abducens, and vestibular cranial nerves nuclei. • Reversible cytotoxic edema is the most distinctive lesion of Wernicke encephalopathy on MRI, represented by symmetric signal abnormality (hyperintense on long-TR images) in the medial thalami, periventricular regions of the third ventricle, mammillary bodies, tectal plate, and periaqueductal area. Signal alteration may also be seen in the cerebellum, cerebellar vermis, cranial nerve nuclei, red nuclei, dentate nuclei, caudate nuclei, splenium, cerebral cortex, and medulla oblongata. Enhancement of the mammillary bodies is occasionally present. • May be associated with parenchymal atrophy, particularly in alcoholic patients. • Prognosis is dependent on time of onset of thiamine supplementation. a. c. b. d. e. Axial T1WI (a.) and T2WI (b-e) demonstrate bilateral symmetric signal abnormality in the medulla oblongata, inferior cerebellar peduncles, tectum, dorsal midbrain, and ventral midbrain in the region of the substantia nigra. There was associated diffusion restriction at the sites of abnormality (not shown). • Progressive mitochondrial neurodegenerative disorder of childhood clinically characterized by psychomotor delay or regression, muscular hypotonia, brainstem signs including strabismus, nystagmus, and swallowing difficulties, ataxia, pyramidal signs, respiratory insufficiency, lactate acidemia, and acute deterioration after common infections. • Neuroimaging findings include focal, bilateral, symmetric necrotic lesions in the basal ganglia, cerebellum, and brainstem which appear hyperintense on T2WI associated with demyelination, vascular proliferation, and gliosis. • Atypical neuroimaging features include diffuse supratentorial leukodystrophy, unifocal or multifocal infarctions, diffuse or focal cortical atrophy, or predominant cerebellar atrophy. • Basal ganglia often affected before the brainstem, and disease typically progresses from upper to lower brainstem. • Involvement of the lower brainstem indicates advanced stage of the disease, with medulla oblongata involvement increasing occurrence of respiratory failure and sudden death. • Bilateral symmetric T2 prolongation involving multiple brainstem structures associated with basal ganglia abnormalities in a child with neurological problems should prompt consideration of Leigh disease. a. b. (a.) Axial T2WI demonstrates flattening of the medulla oblongata (red arrow) with effacement of the surrounding subarachnoid space due to extrinsic compression between the C1-C2 vertebral bodies and low lying cerebellar tonsils within the foramen magnum (yellow arrow). (b.) Sagittal T1WI demonstrates low-lying cerebellar tonsils in a peg-like configuration, consistent with Chiari I malformation. There is indentation on the medulla oblongata due to the extrinsic mass effect. • Congenital craniovertebral junction anomaly characterized by elongated, peg-shaped cerebellar tonsils which extend below the foramen magnum into the upper cervical spinal canal, often associated with crowding of the posterior fossa and compression of the CSF spaces. • May be asymptomatic in up to half of cases. Most common symptoms include headache, neck pain, and ataxia. • When associated with mass effect on the medulla oblongata, may be associated with bulbar symptoms and/or lower cranial nerve palsies. This mass effect may result from direct extrinsic compression by the ectopic cerebellar tonsils, odontoid, or even irregularly coursing posterior inferior cerebellar arteries or vertebral arteries. • Often associated with spinal cord syrinx which may extend to involve the medulla oblongata and cause bulbar symptoms and/or or lower cranial nerve palsies. • Suboccipital decompression is surgical treatment of choice.