* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Exam #2
Biochemical cascade wikipedia , lookup
Restriction enzyme wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
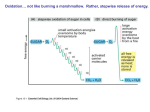
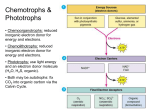
MICRB 201 Study Guide for Exam 2 Content found in Textbook Chapters: 8, 9, 10, and part 11 Mastery of the following is expected, and will help immensely in understanding metabolism and electron transport systems function. I expect a working knowledge. Know the laws of thermodynamics as they apply to metabolic reactions. What is free energy (G); how does the change in free energy (ΔG) determine if a reaction is spontaneous (exergonic), enderogonic, or reversible; what conditions favor negative ΔG? Common concepts: Reversible reactions have ∆G = 0 Exergonic reactions are spontaneous; ∆G < 0; ∆ Eo’ > 0 Endergonic reactions require an energy source (e.g. “burn” an ATP); ∆G > 0; ∆ Eo’ > 0 What is standard reduction potential (Eo’)? For spontaneous redox reactions (exergonic), the relative Eo’ for an electron donor is more negative and that of an electron acceptor is more positive. Know that redox reactions with a ∆ Eo’ < 0 will require extra energy as is the case in ‘reverse electron flow’ for NAD(P)H production in many chemolithotrophs and anoxygenic phototrophs. What happens in anabolism versus catabolism? What is an enzyme? What is the thermodynamic mechanism (activation energy, transition state, enzyme-substrate complex)? Does spontaneity of a reaction relate to the reaction speed? All enzymes have an active site. What is a apoenzyme, cofactor, and holoenzyme? How do the two types of cofactors differ? What is a regulatory (allosteric) site? How can temperature, salt concentration, or pH affect enzyme activity? How does substrate concentration affect enzyme activity. Does an enzymes affinity for substrate (Km value) change with increasing amount of enzyme? Does Vmax increase with increasing amount of enzyme? Be able to explain differences in curves of reaction velocity versus substrate concentration (Michaelis-Menten plots) for the same enzyme from different organisms. What is the difference between competitive versus non-competitive inhibition of enzyme activity. What happens to the substrate affinity of an allosteric enzyme when a negative effector (= noncompetitive inhibitor) is bound; when a positive effector (activator) is bound? What is reversible covalent modification of enzyme activity? What is meant by, “interconvertible enzymes are modified by converter enzymes.” Name the six major classes of enzymes based on reaction type. How can metabolic pathways be organized; how are metabolic pathways controlled? What is feed back inhibition versus feed forward activation? What is an amphibolic pathway. Which enzymes are in common in both directions; which differ? Hydrolysis of complex (polymeric) organics; know which class of enzymes degrade which biological macromolecules; what’s an exoenzyme? Glycolytic Pathway (Embden-Meyerhof); know overall inputs and outputs; what happens in the two stages (Investment and Yield). What is substrate level phosphorylation? Which are the key “pacemaker enzyme” steps. What are the major similarities and differences between Glycolysis and Entner-Doudoroff Pathway? Pentose Phosphate Pathway (PPP); what is its utility for a cell? How is it connected to Glycolysis, at which intermediates? Fermentation as a fate of pyruvate; endogenous organic electron acceptor; why does NADH need to be oxidized; how much energy does fermentation of glucose produce compared to aerobic respiration? What are some fermentation products other than lactic acid and ethanol? Krebs Cycle: what’s the role of the pyruvate dehydrogenase complex (reaction and regulation); what are the products of the Kreb cycle? How would a glycol-lipid be catabolized to CO2 and energy? What is the pathway for fatty acid catabolism and is it an example of a spiral or circular pathway? Electron Transport Chain: How is energy released from reduced electron carriers (NADH, FADH) and what work is performed with it? Chemiosmosis: what is the proton motive force, and how does it relate to ATP Synthase? Why do we call ATP synthesis of this type oxidative phosphorylation (what’s oxidized; what’s phosphorylated)? What is the relationship between the energy yield (P/O ratio value and the change in electron potential between terminal electron acceptor and reduced electron carrier (or other electron donor; e.g. NO2-)? How is aerobic respiration different from anaerobic respiration? How many kind of aerobic respiration are there and which kinds of anaerobic respiration did we discuss? All other growth conditions being identical, why would the growth rate of Escherichia coli decrease from well oxygenated conditions, to low oxygenation, no oxygen with nitrate, to no oxygen nor nitrate? What is dissimilatory nitrate reduction versus denitrification? What are some characteristics of chemolithotrophs; are they always autotrophic? In respiration electrons are donated by endogenous electron carriers (NADH) to an ETC to yield ATP via oxidative phosphorylation; what are the exogenous electron donors of chemolithotrophs for oxidative phosphorylation? Are most chemolithotrophs performing something more similar to aerobic respiration or anaerobic respiration? How efficient is chemolithotrophic energy yield? Why might this relate to having complex internal membrane systems in these cells (see Chapter 3)? Why are they ecologically significant? Name the chemolithotrophs we discussed; what they react with and to what product; any unique differences among them? Why does the energy generated from chemosynthetic electron transport sometimes get shunted to drive reverse electron flow and NAD+ reduction to NADH? (It’s all about Eo’ values) What are the parallels between oxidative phosphorylation and photophosphorylation? What is the difference between chlorophylls and accessory pigments? Name some of each and which bacteria an eukaryotes have them. What is an antenna versus reaction center chlorophyll, versus the photosystem? Cyclic Phosphorylation: what is the flow of electrons? Which phototrophs have this type? Non-Cyclic Phosphorylation: What is the electron donor and what is the electron acceptor? Which phototrophs? How do these two schemes mesh together for green plants and cyanobacteria (Z scheme)? In addition to making ATP, Purple and green bacteria need to make NADH (then NADPH for anabolism). What kinds of molecules donate electrons for this reverse electron flow? What are the basic functions of the Calvin Cycle (inputs and outputs)? What are the three major phases. How is the regeneration phase related to the PPP? How does rhodopsin phototrophy differ from chlorophyll phototrophy (the answer relates to what pumps protons for establishing the PMF)? Explain the potential competitive advantage for survival of photoheterotrophic bacteria in the surface of the open ocean compared to bacteria that are heterotrophs (recall that the open ocean has very low supply of organic nutrients; i.e. it’s oligotrophic). Know where different amphibolic pathway intermediates shunt off for biosynthesis of amino acids, nucleotides, polysaccharides, and lipids. What is the basic premise of the “central dogma” of biology (DNA replication; RNA transcription; protein translation)? What kinds of products result from transcription? Which of the 5 major heterocyclic nitrogenous bases are purines (A,G); which are pyrimidines (C,T,U)? What is the difference between the pentose sugars, ribose and deoxyribose? What is the difference between a nucleoside and a nucleotide? Nucleic acids are nucleotides bound by what kind of bonds? What enzyme catalyzes this reaction? To which end of a growing nucleic acid (5’-P or 3’OH) are nucleotides added? What is base pair complementation? Which bases complement each other in DNA and RNA? Which are stronger complements? What is meant by self-complementation of single stranded nucleic acids (like RNA)? Describe the general structure of double stranded DNA in terms of strand orientation and conformation. Describe DNA replication (what is meant by semi-conservative?) and the enzyme functions involved (DNA Polymerase III, DNA Polymerase I, DNA Gyrase, Helicase, single strand binding (SSB) proteins, primase, ligase. How is leading and lagging strand synthesis different? What is a replisome? How do bacterial chromosomes replicate? What is the oriC, ter, initiation proteins? Explain why it is advantageous to have multiple rrn operons close to the oriC (hint: think fast growth).










![NAME: Quiz #5: Phys142 1. [4pts] Find the resulting current through](http://s1.studyres.com/store/data/006404813_1-90fcf53f79a7b619eafe061618bfacc1-150x150.png)
