* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Poster
Rosetta@home wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein design wikipedia , lookup
Homology modeling wikipedia , lookup
Protein domain wikipedia , lookup
Protein structure prediction wikipedia , lookup
Surround optical-fiber immunoassay wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein folding wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
List of types of proteins wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Western blot wikipedia , lookup
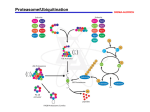
Ubiquitination: The Garbage Cycle of a Cell Grafton High School SMART Team: Dan Burgardt, Lexi Chopp, Ashley Emery, Alyssa Fletcher, Kaleigh Kozak, Adam Schaenzer, Dustin Studelska, Lindsay Wendtlandt, Lindsay Zadra Teacher: Fran Grant Mentor: Namitha Vishveshwara, University of Illinois at Chicago Ubiquitin-Proteasome System Abstract Within every cell, there exists a system known as the ubiquitin-proteasome system (UPS) that eliminates damaged, misfolded or excess proteins. Unwanted proteins are tagged with ubiquitin, a small protein that identifies other proteins as being ready for degradation. The process of activating and transferring the ubiquitin to the protein is referred to as ubiquitination. Three proteins involved in this process are the ubiquitin activating enzyme (E1), the ubiquitin conjugating enzyme (E2), and the ubiquitin ligase (E3). Ubiquitination begins with ubiquitin being activated by and attaching to E1. E1 transfers ubiquitin to E2. Then E2 delivers ubiquitin to the unwanted protein, either directly or through E3. Finally, the tagged protein is broken down by the proteasome, which is the cell’s protein-degrading complex. E2 plays a critical role in the ubiquitination process. Ubch5b is one of many E2s that is involved in tagging unwanted proteins with ubiquitin. Researchers are studying the relationship between the yeast Ubch5b and a specific form of misfolded protein called prions. Prions are unique because when one protein takes the prion form, correctly folded proteins also misfold into prions and aggregate. Prions are infectious proteins; they are not viral or bacterial. Mad cow disease is caused by the presence of prions. In yeast, when Ubch5b is deleted, it leads to increased prion formation. The exact role of the yeast Ubch5b in increased yeast prion formation is still unknown. E2 E1 activates ubiquitin E3 E2 E2 passes ubiquitin on to E3 Activated ubiquitin is transported by E2 E3 E2 Misfolded protein Got Mad Cow? Bovine Spongiform Encephalopathy (BSE), also referred to as "Mad Cow Disease“, is an illness that attacks the brain and spinal cord. The name "Mad Cow Disease" comes from the strange behaviors, including aggression and lack of coordination, that are associated with infected cattle. BSE is the result of prion infection within the brain. Ubiquitin is recycled Take Out the Trash! The Ubiquitin-Proteasome system (UPS) is how the cell rids itself of unwanted, unassembled, or damaged proteins. E3 attaches ubiquitin “tag” to misfolded protein A cow displaying symtoms of Bovine Spongiform Encephalopathy Proteasome Prion Formation Occasionally Normally folded form Misfolded prion form Tagged protein heads for proteasome Proteasome disassembles misfolded protein Efficiently Normally folded form induced into the prion form Prion aggregate A prion is a unique, misfolded form of a normal protein. As illustrated in the diagram above, the prion form initiates a chain reaction by causing other normal proteins of the same type to mis-fold and aggregate together. Though misfolded proteins are targeted to the ubiquitin system (shown in the diagram to the right), the prion escapes this targeting, causing these prion misfolds to accumulate in the cell. Prions are not only present in mammalian cells, but also in yeast cells which has made prion research easier. A SMART Team project supported by the National Institutes of Health (NIH) – National Center for Research Resources Science Education Partnership Award (NCRR-SEPA) What is the connection between the UPS and prions? Ubch5b (in yeast) E2 Increase Normally folded form Prion form In yeast, when Ubch5b(E2) is removed from the ubiqination process, an increase in prion formation has been observed. The easiest explanation would be that a decrease in E2 leads to an increase in misfolded protein, which might lead to increased prion formation. However, as the protein is not normally seen to associate with ubiquitin, the effect of deletion of Ubch5b is probably mediated indirectly.