* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MHP Lab 6 - Transformation and Transcription
Genome evolution wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Non-coding DNA wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Molecular evolution wikipedia , lookup
Gene expression profiling wikipedia , lookup
Community fingerprinting wikipedia , lookup
Gene expression wikipedia , lookup
Point mutation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
List of types of proteins wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene regulatory network wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
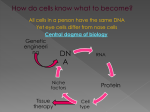
Lab #6 Name: _________________________________________________ Part 1: Bacterial Transformation Part 2: Transcriptional Regulation Part I : Bacterial Transformation INTRODUCTION In this lab you will perform a procedure known as a genetic transformation. Remember that a gene is a piece of DNA that provides the instructions for making (coding for) a protein that an organism requires for appearance or function. Genetic transformation literally means change caused by genes and it involves the insertion of a gene(s) into an organism in order to change the organism's trait(s). Genetic transformation is used in many areas of biotechnology. In agriculture, genes coding for traits such as frost, pest, or spoilage resistance can be genetically transformed into plants. In bio-remediation, bacteria can be genetically transformed with genes enabling them to digest oil spills. In medicine, diseases caused by defective genes are beginning to be treated by gene therapy; that is, by genetically transforming a sick person's cells with healthy copies of the gene involved in their disease. You will use a procedure to transform bacteria with a gene that codes for a Green Fluorescent Protein (GFP). The real-life source of this gene is the bioluminescent jellyfish Aequorea victoria. GFP causes the jellyfish to fluoresce and glow in the dark. Following the transformation procedure, the bacteria express their newly acquired jellyfish gene and produce the fluorescent protein that causes them to glow a brilliant green color under ultraviolet light. In this activity, you will learn about the process of moving genes from one organism to another with the aid of a plasmid. In addition to one large chromosome, bacteria naturally contain one or more small circular pieces of DNA called plasmids. Plasmid DNA usually contains genes for one or more traits that may be beneficial to bacterial survival. In nature, bacteria can transfer plasmids back and forth, which creates the opportunity for them to share these beneficial genes. (Note that the bacteria don’t know that they are picking up beneficial genes.) This natural mechanism allows bacteria to adapt to new environments. The recent occurrence of bacterial resistance to antibiotics is due to the transmission of plasmids. You will be provided with the tools and a protocol for performing genetic transformation in Escherichia coli. This transformation procedure involves three main steps. These steps are intended to introduce the plasmid DNA into the E. coli cells and provide an environment for the cells to express their newly acquired genes. Many species of bacteria have special membrane proteins for the uptake of DNA from the external environment. E. coli does not appear to have these types of membrane proteins; however, placing E. coli in a relatively high concentration of calcium ions and performing a procedure called “heat shock” will stimulate these cells to take up pieces of foreign DNA. Read the lab exercise and follow the directions carefully. Completion of the lab will take 2 lab periods (or 1 lab and 1 class). In the second lab period you will analyze your results. You will practice using sterile technique, as instructed at the beginning of lab session, before you do the experiment. When culturing bacteria, you must not introduce other, contaminating bacteria into your culture. Potentially contaminating bacteria are ubiquitous; they are found everywhere (including on the bench top and on your hands). It is especially important to keep the inoculation loops, the pipette tips, and the surfaces of the agar plates must not touch or be touched by any contaminating surface. PURPOSE To transform bacteria with plasmid DNA and analyze growth on agar plates containing either ampicillin alone or ampicillin and arabinose. MATERIALS AND PROCEDURES 1. Follow the procedures in the “Transformation Kit-Quick Guide” provided in lab. 2. The plates will be incubated for 24-48 hours, and then placed in a refrigerator to slow the growth of the bacteria. You will than observe the plates in the next lab period to collect your data. 3. Complete your lab report: Answer the questions and fill in the table in the space provided below. Complete the Hypothesis, Predictions, and Experimental Design sections during the first lab period. The Results section will be completed after we analyze the data next week. PRE-LAB QUESTIONS What is ampicillin? What is arabinose? What are they used for when growing bacteria on LB-agar plates? NOTE: LB is the nutrient mixture that is added to the plate agar to feed the bacteria. Based on the above answers, prepare and complete the table below to indicate what you predict will happen on each of the four agar plates. (Will E. coli grow on these plates? Will the E. coli have any special properties compared to wild type?) Plate Plasmid? LB/amp LB/amp/ara LB/amp LB +DNA +DNA -DNA -DNA Growth (G) No Growth (NG) Other properties? 1. What is/are the independent variable(s) in this experiment? 2. What is/are the dependent variable(s)? 3. Which plates will serve as control plates? Do you expect cells to grow on these plates? Why or why not? What is the purpose of these controls? 4. Define plasmid. Part 2: Transcriptional Regulation INTRODUCTION We know that DNA is the genetic material for life. DNA is transcribed to make RNA, and the RNA serves as a template for protein synthesis. In the class, you are learning that this description of the process is over-simplified. In fact, there is regulation of every step in the process, right down to how long the protein remains stable inside the cell. In this section, we are going to examine how transcription can be regulated – How does the cell decide if RNA will be made from a particular gene template. Although we will not do the actual experiments to test transcriptional regulation in a eukaryotic system, you will design a “mock” experiment and I will provide you with data to analyze. The scenario: Imagine you are working in a lab. The student who was working there before you discovered a new gene – NSCC1 (Figure 1). She cloned the whole coding sequence (dark blue box), as well as the upstream promoter region (left of the blue box). The promoter region of a gene is what determines whether or not a gene will be used to synthesize RNA. The process of synthesizing RNA based on a gene template is called “gene expression”. The promoter region contains specific sequences that bind proteins known as transcription factors. Binding of transcription factors to the promoter signals the proteins that transcribe all genes to express that particular gene (see Chapter 17 of your textbook). After sequencing the DNA clone, the student found 3 E-box sequences in the promoter (light blue boxes). The sequence of an E-box is CANNTG, where the Ns can be any nucleotide. The interesting part about this sequence is that it may bind to transcription factors found only in skeletal muscle cells, such as MyoD. Your task is to figure out whether or not this is the case for this particular gene. Figure 1 – The new gene. possible E-boxes transcriptional start site TATA box unknown gene – NSCC1 promoter In order to determine this, you first ligated the promoter sequence of the new gene into the pGL2 reporter vector, shown in Figure 2 (don’t worry about the details of this – we will come back to it in the DNA Technology section). So, now your promoter sequence is upstream of the luciferase gene in the plasmid, instead of NSCC1. These plasmids can be transfected into mammalian cells using specific transfection reagents. If the cells that you transfect have the transcription factors that bind to the promoter region, and these factors can bind to the plasmid DNA, they will activate transcription of the luciferase gene, thus making luciferase protein. Luciferase is an enzyme from fireflies that catalyzes the breakdown of luciferin to generate light. After the cells are transfected, the proteins in the cells are given time to find the plasmid and activate transcription of the luciferase gene. Protein is isolated from the cells - the amount of protein isolated can be measured using a specific protein quantitation reagent and a small sample of the isolate. The activity of the luciferase enzyme is measured by adding luciferin to a sample of the protein extract and measuring the light emitted using a luminometer. The amount of luciferase activity is directly related to how well the promoter is able to activate expression of the luciferase gene – promoter binds transcription factors, gene is expressed (RNA is made), RNA is made into protein. You have three cell types available to you – skeletal muscle cells (C2C12 cells), kidney cells (COS-1) and fibroblasts (3T3). The skeletal muscle cells have muscle-specific transcription factors such as MyoD. MyoD can bind to the E-box sequence and activate transcription of certain muscle-specific genes. COS-1 kidney cells and 3T3 fibroblasts do not have the MyoD transcription factor and therefore do not activate transcription of these muscle-specific genes. Keep in mind that you have a plasmid that will make MyoD protein in fibroblasts. Figure 2 – pGL3 reporter plasmid. luciferase polyA promoter ampicillin resistance MATERIALS C2C12 cells COS-1 cells 3T3 cells (assume you have all the things available to grow the cells) Tranfection reagent pGL3 plasmid pGL3 plasmid containing the promoter EMSV plasmid containing MyoD cDNA (can express in fibroblasts) Protein extraction buffer Luciferin Luminometer Protein quantitaton reagent Spectrophotometer (assume you have tubes available to you) PROCEDURE Let us begin with the hypothesis that the gene will only be expressed in muscle cells. How are you going to test that hypothesis? Make sure you have appropriate negative controls. DATA AND OBSERVATIONS The data will be given to you during the laboratory session. After discussing the procedures that were developed, and the data distributed, we will discuss what to do with the data. To hand in: The report will be due on Thursday August 3rd. You will hand in the predictions and answered questions from Part 1. You will also hand in the outline of the procedure that you developed for Part 2. Lastly, you will hand in the data analysis from Part 2, including a graph of the results and your conclusions.