* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell Structure and Function
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Tissue engineering wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
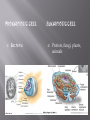
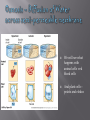
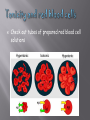
BSC 2010 L Cells are the basic unit of life Cell Theory All living things are composed of cells and Cells come only from other cells In this lab, we will look at a couple different things: Cell Structure Plasma membranes Difference between prokaryotes and eukaryotes Eukaryotes – difference between plant and animal cells Function of organelles Contents of the cell are bounded by plasma membrane Regulates movement of molecules into and out of the cytoplasm We will look at the how the passage of water into a cell depends on the difference in concentration of solutes between cytoplasm and the surrounding medium pH Well-being of cells also depends upon pH surrounding them We will look at how a buffer can maintain pH within a narrow range and how buffers within cells can protect them from damaging pH changes PROKARYOTIC CELL Bacteria EUKARYOTIC CELL Protists, fungi, plants, animals Know the name and function of organelles found in eukaryotic cells Know differences between plant cells and animal cells! Looking at the Elodea Leaf Human Cheek Cells pH of a solution tells its hydrogen ion concentration H+ Buffer – system of chemicals that takes up excess H ions or hydroxide ions Maintaining pH is VERY important in biological systems i.e. – human blood pH must be maintained at 7.4 Exercise 4.5 You will test the buffering capacity of 3 solutions Water, inorganic solution, simulated cytoplasm Which one do you think will be able to hold its pH the longest as drops of HCl are added? Contents pH before acid pH after acid Additional acid ~ 6.5 3.0 2.0 Inorganic Buffer 7.0 7.0 5.5 Cytoplasm 7.0 7.0 6.5 Distilled Water Movement of molecules from a higher to a lower concentration until equilibrium is achieved Dependent on temperature, size of molecules, and type of medium (solid, semi-solid, liquid, air) Air Liquid Semi-solid Semi-permeable membrane Dialysis tubing Diffusion of methylene blue in agar (semisolid) versus water (liquid) In which medium did the dye diffuse the fastest? We will see what happens with animal cells -red blood cells And plant cells – potato and elodea Relative concentration of solute (particles) or solvent (water) outside the cell compared to inside 3 Types Isotonic Hypertonic Hypotonic Check out tubes of prepared red blood cell solutions Isotonic Hypertonic In 10% NaCl solution Water has left the cell across the plasma membrane Notice how cell membrane has shrunk away from cell wall and chloroplasts are all clustered in middle