* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 29
Lipid signaling wikipedia , lookup
Point mutation wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Signal transduction wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Butyric acid wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Genetic code wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Citric acid cycle wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
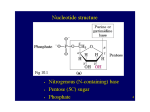
Lecture 29 Purine Metabolism/Diseases Raymond B. Birge, PhD Nucleotides - key roles in cellular processes: 1. Activated precursors of RNA and DNA 2. Adenine nucleotides are components of the major co-enzymes, NAD, NADP, FMN, FAD, and CoA 3. Nucleotide derivatives are activated intermediates in biosynthetic processes (UDP-glucose, SAM) 4. Serve as metabolic regulators (e.g cAMP and the activation of cell signaling). 5. Serve as major currency of energy in all cells (ATP and GTP). 6. Several metabolic diseases have their etiology in nucleotide metabolism. Purine metabolism (Overview) 1. Nomenclature/nucleotide structure 2. De novo synthesis pathways 3. Re-utilization (salvage) pathways 4. Degradation pathways 5. Metabolic diseases of purine metabolism (Gout, Lesch-Nyhan, SCID) Suggested reading: Lippencott’s Chapter 22 Nomenclature Adenine BASE Adenosine Adenosine monophosphate (AMP) NUCLEOSIDE NUCLEOTIDE Active forms of nucleotides: di-and tri-phosphates Nucleoside Monophosphate Kinase (i) GMP + ATP (ii) Nucleoside Diphosphate Kinase XDP + YTP GDP + ADP XTP + YDP Why are nucleosides and nucleotides important For biochemists? Purine binding proteins (“the purine proteome”) comprise a family of 3-4,000 Proteins and as much as 50% of all druggable targets in biology. Kinases Helicases Reductases Transferases Synthetases Dehydrogenases Chaperones Metabolic Enzymes DNA and RNA processing Etc Common Purine Bases O NH2 Adenine Hypoxanthine O O O NH2 Xanthine H= 6 oxy purine X= 2,6 dioxy purine Guanine Guanine A= 6 amino purine G= 2 amino, 6-oxy purine Nucleoside Function in extracellular signal transduction Adenosine nucleoside-increased during ATP degradation. Released in cells when there is low O2 concentration Binds to purinogenic receptors A1, A2A, A2B, A3 Slows the heart down, at the same time increases capillary dilation Caffeine is a adenine derivative, and antagonizes the effects of adenine. Cyclic nucleotides are important mediators for Intracellular signal transduction 10-10 M cAMP ATP PKA P phosphorylase kinase P phosphorylase P glycogen synthase Two pathways to generate purines and pyrimidines 1. DE NOVO BIOSYNTHETIC PATHWAYS (building the bases from non-purine molecules) 2. SALVAGE PATHWAYS (the reutilization of bases from dietary or catabolic sources) De novo biosynthesis of purines (A and G): activation of ribose-phosphate Pentose phosphate pathway IMP, AMP, GMP Ribose phosphate pyrophosphoKinase (PRPP synthase) Major regulatory step in purine biosynthesis: PRPP to 5-Phosphoribosyl-1-amine Glutamine Glutamate PRPP * PPi Amidophosphoribosyl transferase Inhibited by products IMP, AMP, and GMP. FEEDBACK INHIBITION Purine biosynthesis intermediates Purines: where do the atoms come from? Key: Glycine 1 C unit of N10 f-TetraHydroFolic acid (sulfonamides; methotrexate) Glutamine Asparate Amino acids utilized in purine biosynthesis Inosine monophosphate is the precursor for both AMP and GMP AMP & GMP synthesis IMP Hypoxanthine to Adenine/Guanine. O NH2 (N source) Aspartate Hypoxanthine-IMP Adenine O O (N source) Glutamine O Xanthine-XMP NH2 Guanine The common mechanistic theme for the conversion to A and G is the conversion of a carbonyl oxygen to an amino group Regulation of purine de novo biosynthesis: classic negative feedback Ribose 5-phosphate PRPP Phosphoribosyl amine Inhibited by AMP Inhibited by IMP, AMP, and GMP AMP IMP GMP Inhibited by GMP Salvage pathways: re-utilizate purines O O P O CH2 OH OH O OH PPi + Base (Adenine or Guanine) PRPP + Adenine PRPP + Guanine O O P O CH2 OH A-PRT HG-PRT OH A O + PPi OH Adenylate/AMP Guanylate/GMP There are 2 salvage enzymes with different specificities: 1. Adenine phosphoribosyl transferase (APRT) 2. Hypoxanthine-guanine phosphoribosyl transferase (HGPRT) Stages of nucleotide metabolism Endonuclease Nucleic Acid Synthesis Phosphodiesterase Nucleoside monophosphates (Mononucleosides) Nucleoside triphosphate Endonuclease Nucleic Acid Synthesis Phosphodiesterase Nucleotidases H20 Nucleoside monophosphates (Mononucleosides) Pi Nucleoside triphosphate PPi ADP Nucleosides Nucleoside kinase ATP Phosphoribosyl transferases Pi PRPP Phosphorylases Ribose-1-P Nucleobases (A, G) Uric Acid (purines) Getting Back to ‘Basics’ Nucleotidase Adenine Phosphorylase Adenosine monophosphate (AMP) Adenosine NUCLEOTIDE NUCLEOSIDE BASE Purines in humans are degraded to urate (ADA) Gouty Arthritis The Gout James Gilray 1799 By Royal Authority ‘King George IV’ George Cruickshank 19th C. Some famous people who had gout Henry VIII Kublai Khan Nostradamus John Milton Isaac Newton Frederick the Great John Hancock Thomas Jefferson Benjamin Franklin David Wells Gout results from HYPERURICEMIA Decreased URIC ACID excretion: 80% of gout ideopathic, renal disease, diabetes insipidus, hypertension, Downs syndrome, many others Increased URIC ACID production: 20% of gout – PRPP synthase overactivity, hemolytic diseases, lymphoproliferative diseases, may others HGPRT deficiency (Lesch Nyhan Syndrome), exacerbated by alcohol, purine rich diet, obesity Gout from increased uric acid production 1. Lost regulation of PRPP Synthase & PRPP Amidotransferase Ribose 5-phosphate PRPP Phosphoribosyl amine purines Inhibited by IMP, AMP, and GMP Leads to net increase in biosynthetic/degradation pathways!! (From slide #18) Gout from increased uric acid production 2. Defects in salvage pathway lead to increased PRPP & Guanine PRPP + Guanine [HG-PRT] GMP Leads to net increase in biosynthetic/degradation pathways!! GOUT Treatment Hypoxanthine Guanine xanthine oxidase Xanthine Urate xanthine oxidase NaUrate crystals in PMLs Allopurinol: a. decrease urate b. increase xanthine & hypoxanthine c. decrease PRPP Tophi at helix of ear 1st MTP joint Initiate urate lowering therapy !! Large tophaceous deposits surrounding joint Clinical Significance of Purine metabolism ID: A 56 year old obese white male comes to his family doctor. Chief Complaint: ‘My big toe hurts like !*?!#*!?!!!’ History Present Illness: Pain began during the night after an episode of binge eating and drinking. Past Medical History: Significant for removal of kidney stones last year. Current Health/Risk Factors: He admits to being an avid meat eater and drinks beer every night. Physical Exam: fever, right metatarsophalangial (MTP) joint red, hot and swollen; painful to motion; subcutaneous deposits in helix of left ear Pathology: Synovial fluid aspiration shows negatively birefringent, needle-shaped crystals. Lesch-Nyhan Syndrome X-linked recessive Severe HGPRT deficiency: decreased IMP&GMP increased PRPP & de novo purine p’way Hyperuremia: gouty arthritis, kidney stones, tophi Neurologic disability: spasticity, hyperreflexia Behavioral problems: cognitive dysfunction, aggression, self-injury SCID Severe Combined Immunodeficiency Syndrome Autosomal recessive disorder Mutations in ADA* AMP H20 Nucleotidase Pi Infants subject to bacterial, candidiasis, viral, protazoal infections Adenosine H20 NH3 Inosine Adenosine deaminase* Hypoxanthine Both T and B cells reduced (dATP is toxic) 1995-AdV expressing ADA was successfully employed as gene therapy strategy Adenosine Deaminase (ADA) deficiency Urate (From slide #19) Bottom Line Recognize names and structures of purines/nomenclature of NMPs: Adenosine, Guanine, Hypoxanthine, Xanthine Name the precursors of atoms in the purine ring: Gln (N); Gly (C, N); N10-fTHF (C ); HCO3- (CO); Asp (N) Recognize the regulated reactions: PRPP synthase: IMP, AMP, GMP Gln:PRPP amidotransferase: IMP, AMP, GMP; PRPP IMP AMP: AMP IMP GMP: GMP Explain the cause of SCIDS Make differential diagnosis of gouty arthritis and Lesch-Nyhan Syndrome Summary and Take-Home Points 1. Identify basic structures of purines, nucleosides, and nucleotides. 2. Identify key relationships between glucose metabolism and purine biosynthesis. 3. Knowledge of how amino acids are used in AMP and GMP biosynthesis. 4. Understand degradation pathways of purines and their relationship to uric acid metabolism and gout Integrative Thought Question Ribonucleotides and Deoxyribonucleotides are essential for all cells, and represent key convergent points in energy metabolism. Provide specific examples in which purine metabolism can be linked to metabolism of 1). Glucose 2). Lipids 3). Amino Acids 4). Ammonium