* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Figure 4-24, step 1
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Proteolysis wikipedia , lookup
Electron transport chain wikipedia , lookup
Gene expression wikipedia , lookup
Citric acid cycle wikipedia , lookup
Messenger RNA wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
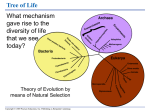
POWERPOINT® LECTURE SLIDE PRESENTATION by LYNN CIALDELLA, MA, MBA, The University of Texas at Austin UNIT 1 4 Energy and Cellular Metabolism HUMAN PHYSIOLOGY AN INTEGRATED APPROACH DEE UNGLAUB SILVERTHORN Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings FOURTH EDITION About this Chapter Energy in biological systems Chemical reactions Enzymes Metabolism ATP production Synthetic pathways Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Energy: Biological Systems Energy transfer in the environment Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-1 Energy: Capacity to Do Work Chemical work Making and breaking of chemical bonds Transport work Moving ions, molecules, and larger particles Creates concentration gradients Mechanical work Used for movement Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Energy: Two Forms The relationship between kinetic energy and potential energy Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-2a Energy: Two Forms Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-2b Energy: Two Forms Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-2c Energy: Thermodynamics First law of thermodynamics Total amount of energy in the universe is constant Second law of thermodynamics Processes move from state of order to disorder or entropy Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Reactions: Overview Activation energy is the energy that must be put into reactants before a reaction can proceed A+BC+D Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-3 Chemical Reactions: Coupling Energy transfer and storage in biological reactions Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-5 Enzymes: Overview Speed up the rate of reactions Isozymes Catalyze same reaction but under different conditions May be activated, inactivated, or modulated Coenzymes vitamins Chemical modulators temperature and pH Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Enzymes: Speed Up Reactions Enzymes lower the activation energy of reactions Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-8 Enzymes: Law of Mass Action Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-9a Enzymes: Law of Mass Action Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-9b Enzymes: Types of Reactions Types 1. Oxidation-reduction 2. Hydrolysis-dehydration 3. Addition-subtractionexchange 4. Ligation Description +/- electrons or H+ +/- water +/- or exchange groups Joins using ATP Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Metabolism: Overview A group of metabolic pathways resembles a road map Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-10 Metabolism: Cell Regulation Controlling enzyme concentrations Producing modulators Feedback inhibition Using different enzymes Isolating enzymes Maintaining ratio of ATP to ADP ADP + Pi + energy ATP Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings ATP Production: Overview Overview of aerobic pathways for ATP production Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-13 ATP Production: Glycolysis Glucose + 2 NAD+ + 2 ADP + P 2 Pyruvate + 2 ATP + 2 NADH + 2 H+ + 2 H20 Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-14 ATP Production: Pyruvate Metabolism Pyruvate can be converted into lactate or acetyl CoA Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-15 ATP Production: Citric Acid Cycle Acetyl CoA enters the citric acid cycle producing 3 NADH, 1 FADH2, and 1 ATP Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-16 ATP Production: Electron Transport Mitochondrial matrix CITRIC ACID CYCLE 2 H2O e– O2 + Inner mitochondrial membrane Matrix pool of H+ 3 1 ATP 4e– High-energy electrons 2 H+ H+ ADP + Pi 4 H+ H+ H+ H+ Intermembrane space H+ High-energy electrons Electron transport system Outer mitochondrial membrane High-energy electrons from glycolysis 1 Energy released during metabolism is captured by highenergy electrons carried by NADH and FADH2. 2 Energy from high-energy electrons moving along the protein complexes of the electron transport system pumps H+ from the matrix into the intermembrane space. Cytosol 3 Electrons at the end of the electron transport system are back to their normal energy state. They combine with H+ and oxygen to form water. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings 4 Potential energy captured in the H+ concentration gradient is converted to kinetic energy when H+ pass through the ATP synthase. Some of the kinetic energy is captured as ATP. Figure 4-17 ATP Production: Electron Transport CITRIC ACID CYCLE Mitochondrial matrix Inner mitochondrial membrane e– 1 High-energy electrons Intermembrane space High-energy electrons Electron transport system Outer mitochondrial membrane High-energy electrons from glycolysis Cytosol 1 Energy released during metabolism is captured by highenergy electrons carried by NADH and FADH2. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-17, step 1 ATP Production: Electron Transport Mitochondrial matrix CITRIC ACID CYCLE Inner mitochondrial membrane e– 1 e– High-energy electrons 2 H+ H+ H+ Intermembrane space H+ H+ H+ High-energy electrons Electron transport system Outer mitochondrial membrane High-energy electrons from glycolysis Cytosol 1 Energy released 2 Energy from high-energy during metabolism electrons moving along is captured by highthe protein complexes energy electrons of the electron transport carried by NADH system pumps H+ from and FADH2. the matrix into the intermembrane space. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-17, steps 1–2 ATP Production: Electron Transport Mitochondrial matrix CITRIC ACID CYCLE 2 H2O e– Inner mitochondrial membrane O2 + Matrix pool of H+ 3 1 4e– High-energy electrons 2 H+ H+ H+ Intermembrane space H+ H+ H+ High-energy electrons Electron transport system Outer mitochondrial membrane High-energy electrons from glycolysis 1 Energy released 2 Energy from high-energy during metabolism electrons moving along is captured by highthe protein complexes energy electrons of the electron transport carried by NADH system pumps H+ from and FADH2. the matrix into the intermembrane space. Cytosol 3 Electrons at the end of the electron transport system are back to their normal energy state. They combine with H+ and oxygen to form water. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-17, steps 1–3 ATP Production: Electron Transport Mitochondrial matrix CITRIC ACID CYCLE 2 H2O e– Inner mitochondrial membrane O2 + Matrix pool of H+ 3 1 ATP 4e– High-energy electrons 2 H+ H+ ADP + Pi 4 H+ H+ H+ H+ Intermembrane space H+ High-energy electrons Electron transport system Outer mitochondrial membrane High-energy electrons from glycolysis 1 Energy released 2 Energy from high-energy during metabolism electrons moving along is captured by highthe protein complexes energy electrons of the electron transport carried by NADH system pumps H+ from and FADH2. the matrix into the intermembrane space. Cytosol 3 Electrons at the end of the 4 Potential energy captured in the H+ concentration gradient electron transport system is converted to kinetic energy are back to their normal when H+ pass through the ATP energy state. They combine + synthase. Some of the kinetic with H and oxygen to energy is captured as ATP. form water. NADH and FADH2 ATP by oxidative phosphorylation Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-17, steps 1–4 ATP Production: Large Biomolecules Glycogenolysis Glycogen Storage form of glucose in liver and skeletal muscle Converted to glucose or glucose 6-phosphate Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings ATP Production: Large Biomolecules Protein catabolism and deamination Catabolism Hydrolysis of peptide bonds Deamination Removal of amino group Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings ATP Production: Lipolysis If acetyl CoA production exceeds capacity for metabolism, production of ketone bodies results Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-20 Synthesis: Gluconeogenesis Glucose can be made from glycerol or amino acids in liver and kidney Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-21 Synthesis: Lipids Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-22 Synthesis: Lipids Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-22 (1 of 3) Synthesis: Lipids Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-22 (2 of 3) Synthesis: Lipids Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-22 (3 of 3) Synthesis: Lipids Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-22 Synthesis: Protein The major steps required to convert the genetic code of DNA into a functional protein 20 different amino acids made from 4 nitrogenous bases Gene 1 GENE ACTIVATION Regulatory proteins Constitutively active Regulated activity Induction Repression 2 TRANSCRIPTION mRNA 3 mRNA PROCESSING Alternative splicing Processed mRNA siRNA Interference mRNA “silenced” • rRNA in ribosomes • tRNA • Amino acids 4 TRANSLATION Nucleus Cytoplasm Protein chain 5 POST-TRANSLATIONAL Folding and Cleavage into Addition of groups: Assembly into MODIFICATION • sugars cross-links smaller peptides polymeric • lipids proteins • —CH3 • phosphate Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24 Synthesis: Protein Gene 1 GENE ACTIVATION Constitutively active Regulatory proteins Regulated activity Induction Repression Nucleus Cytoplasm Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24, step 1 Synthesis: Protein Gene 1 GENE ACTIVATION Constitutively active Regulatory proteins Regulated activity Induction Repression 2 TRANSCRIPTION mRNA Nucleus Cytoplasm Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24, steps 1–2 Synthesis: Protein Gene 1 GENE ACTIVATION Regulatory proteins Constitutively active Regulated activity Induction Repression 2 TRANSCRIPTION mRNA 3 mRNA PROCESSING Alternative splicing Processed mRNA siRNA Interference mRNA “silenced” Nucleus Cytoplasm Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24, steps 1–3 Synthesis: Protein Gene 1 GENE ACTIVATION Regulatory proteins Constitutively active Regulated activity Induction Repression 2 TRANSCRIPTION mRNA 3 mRNA PROCESSING Alternative splicing Processed mRNA siRNA Interference mRNA “silenced” • rRNA in ribosomes • tRNA • Amino acids 4 TRANSLATION Nucleus Cytoplasm Protein chain Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24, steps 1–4 Synthesis: Protein Gene 1 GENE ACTIVATION Regulatory proteins Constitutively active Regulated activity Induction Repression 2 TRANSCRIPTION mRNA 3 mRNA PROCESSING Alternative splicing Processed mRNA siRNA Interference mRNA “silenced” • rRNA in ribosomes • tRNA • Amino acids 4 TRANSLATION Nucleus Cytoplasm Protein chain 5 POST-TRANSLATIONAL Folding and Cleavage into Addition of groups: Assembly into MODIFICATION • sugars cross-links smaller peptides polymeric • lipids proteins • —CH3 • phosphate Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-24, steps 1–5 Protein: Transcription Transcription factors bind and activate promoter region RNA polymerase binds and “unwinds” DNA mRNA created from sense strand mRNA is processed by RNA interference Alternative splicing Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Protein: Transcription and Translation DNA 1 Transcription 2 RNA polymerase mRNA processing Nuclear membrane Amino acid tRNA 4 Translation Growing peptide chain Incoming tRNA bound to an amino acid Lys Asp Outgoing “empty” tRNA 3 Attachment of ribosomal subunits Phe Trp Anticodon mRNA AA G A C C G AU UU C UG G A A A Ribosome mRNA 5 Termination Ribosomal subunits Completed peptide Each tRNA molecule attaches at one end to a specific amino acid. The anticodon of the tRNA molecule pairs with the appropriate codon on the mRNA, allowing amino acids to be linked in the order specified by the mRNA code. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27 Protein: Transcription and Translation DNA 1 Transcription RNA polymerase Nuclear membrane Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27, step 1 Protein: Transcription and Translation DNA 1 Transcription 2 mRNA processing RNA polymerase Nuclear membrane Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27, steps 1–2 Protein: Transcription and Translation DNA 1 Transcription 2 mRNA processing RNA polymerase Nuclear membrane 3 Attachment of ribosomal subunits Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27, steps 1–3 Protein: Transcription and Translation DNA 1 Transcription 2 mRNA processing RNA polymerase Nuclear membrane Amino acid tRNA 4 Translation Growing peptide chain Incoming tRNA bound to an amino acid Lys Asp 3 Attachment of ribosomal subunits Outgoing “empty” tRNA Phe Trp Anticodon mRNA AA G A C C G AU UU C UG G A A A Ribosome Each tRNA molecule attaches at one end to a specific amino acid. The anticodon of the tRNA molecule pairs with the appropriate codon on the mRNA, allowing amino acids to be linked in the order specified by the mRNA code. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27, steps 1–4 Protein: Transcription and Translation DNA 1 Transcription 2 RNA polymerase mRNA processing Nuclear membrane Amino acid tRNA 4 Translation Growing peptide chain Incoming tRNA bound to an amino acid Lys Asp Outgoing “empty” tRNA 3 Attachment of ribosomal subunits Phe Trp Anticodon mRNA AA G A C C G AU UU C UG G A A A Ribosome mRNA 5 Termination Ribosomal subunits Completed peptide Each tRNA molecule attaches at one end to a specific amino acid. The anticodon of the tRNA molecule pairs with the appropriate codon on the mRNA, allowing amino acids to be linked in the order specified by the mRNA code. Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-27, steps 1–5 Protein: Post-Translational Modification Protein folding Creates tertiary structure Cross-linkage Strong covalent bonds disulfide Cleavage Addition of other molecules or groups Assembly into polymeric proteins Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Protein: Post-Translational Modification and the Secretory Pathway Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Figure 4-28 Summary Energy Chemical Transport Mechanical work Kinetic energy Potential energy Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Summary Chemical reactions Reactants Products Reaction rate Free energy and activation energy Exergonic versus endergonic reactions Reversible versus irreversible reactions Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Summary Enzymes Definition Characteristics Law of mass action Type of reactions Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Summary Metabolism Catabolic versus anabolic reactions Control of metabolic pathways Aerobic versus anaerobic pathways Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Summary ATP production Glycolysis Pyruvate metabolism Citric acid cycle Electron transport chain Glycogen, protein, and lipid metabolism Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Summary Synthetic pathways Gluconeogenesis Lipid synthesis Protein synthesis Transcription Translation Post-translational modification Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings