* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download here
Promoter (genetics) wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Gene expression profiling wikipedia , lookup
Molecular evolution wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Community fingerprinting wikipedia , lookup
Magnesium transporter wikipedia , lookup
DNA vaccination wikipedia , lookup
Interactome wikipedia , lookup
Gene expression wikipedia , lookup
Western blot wikipedia , lookup
Protein adsorption wikipedia , lookup
Gene nomenclature wikipedia , lookup
Point mutation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene regulatory network wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Proteolysis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein purification wikipedia , lookup
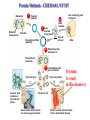
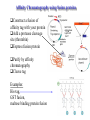
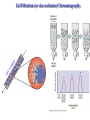
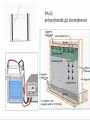
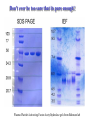
Protein Methods – CHEM 641, 9/17/07 1 Bacterium Plasmid isolated 2 DNA isolated 3 Gene Bacterial chromosome Cell containing gene of interest inserted into plasmid Plasmid Gene of interest Recombinant DNA (plasmid) 4 DNA Plasmid put into bacterial cell Recombinant bacterium 5 Cell multiplies with gene of interest Copies of gene Gene for pest resistance inserted into plants Copies of protein Clones of cell Gene used to alter bacteria for cleaning up toxic waste Proteins to study in Biochemistry Protein used to make snow form at higher temperature Protein used to dissolve blood clots in heart attack therapy Overview of Prokaryotic Expression Strong promoter – Plac Ribosome binding – Shine-Dalgarno sequence ~ 7 b.p. before start codon: AUG Multicloning site to put your gene in with correct frame and direction. Affinity Chromatography using fusion proteins Construct a fusion of affinity tag with your protein Add a protease cleavage site (thrombin) Express fusion protein Purify by affinity chromatography Cleave tag Examples: His-tag, GST fusion, maltose binding protein fusion Gel Filtration (or size exclusion) Chromatography Ion Exchange Chromatography Protein’s isoelectric point Blue – pos. Red – neg. Yellow - polar http://binfo.ym.edu.tw/bioflash/emboss/iep/iep.htm SDS PAGE SDS-sodium docecylsulfate Denaturing conditions Boil 100 ºC DTT, b-mercaptoethanol Cys-S-S-Cys Cys-SH Elution rate to log MW MWM crude fusion cleaved protein of interest Don’t ever be too sure that its pure enough! Plasma Platelet Activating Factor Acetylhydrolase gels from Bahnson lab 2D PAGE IEF followed by SDS PAGE Homogeneity / Heterogeneity A. Post translational modification – examples: phosphorylation, glycosylation, myristoylation B. Chemical modifications – cysteine oxidation, Asn/Gln hydrolysis C. Aggregation, unfolding D. Order / disorder E. Alternate Conformations – example hemoglobin bound vs. unbound with oxygen Next class • Protein Structure Determination: X-ray Crystallography, NMR Spectroscopy and Homology Modeling • Reading: pgs 136-139 Lehninger • http://www.udel.edu/chem/bahnson/Chem6 41/Protein-Structure-and-Function.pdf • Class slides: go to CHEM 641 links page