* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download downloading
Extracellular matrix wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
List of types of proteins wikipedia , lookup
Cellular differentiation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Wnt signaling pathway wikipedia , lookup
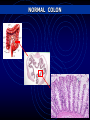
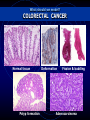
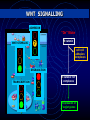
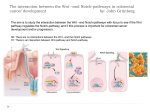
MULTISCALE MODELLING OF COLONIC CRYPTS AND EARLY COLORECTAL CANCER Helen Byrne Computational Biology Group, Computer Science and OCCAM [email protected] Oxford, January 2012 OUTLINE • Motivation • Subcellular model of Wnt signalling • Multiscale modelling of CRC • Continuum modelling • Discussion MOTIVATION Why CRC? Prevalence 1. 2. 3. 4. 5. Lung: Breast: Colon: Stomach: Prostate: incidence mortality 1 238 000 1 050 000 943 000 875 000 542 000 1 102 372 491 646 204 000 000 000 000 000 Worldwide each year NORMAL COLON Colorectal crypts Paul Appleton, Dundee • • • Crypts of Lieberkühn in the small intestine Limited number of cells (~700) Human crypts self-renew every 5-7 days NORMAL COLON Absorptive columnar cell Mucinous Goblet cell Why model CRC? Well-characterised sequence of mutations APC Normal epithelium Hypomethylation Hyperproliferative epithelium Early adenoma K-RAS Intermediate adenoma Fearon and Vogelstein (1990) “A genetic model for colorectal carcinogenesis” Cell 61, 759-767 DCC? Late adenoma p53 Other alterations Carcinoma Metastasis Note: progression to cancer is not unique! GENETIC ALTERATIONS What should we model? COLORECTAL CANCER Normal tissue Polyp formation Deformation Fission & budding Adenocarcinoma What should we model? NORMAL CRYPT RENEWAL CYCLE 5 1. Proliferation of stem cells (bottom of crypt) 2. Progeny divides a few times (lower third of crypt) 3. Progeny starts migrating to the surface 4. Progeny differentiates (midcrypt region) 5. Senile cells are removed from the surface (midpoint between crypts) How are these processes coordinated? 3 4 2 1 WNT SIGNALLING Co-ordination of cell proliferation, migration and differentiation: WNT SIGNALLING Response to Wnt signalling is mediated by b-catenin and APC MUTATIONS IN THE WNT PATHWAY • 12% of CRCs have a mutation in βcatenin mutation, rendering β-catenin insensitive to APC complex • 80% have a double APC mutation, rendering APC complex ineffective Mutations in Wnt pathway early event in CRC APC Normal epithelium Hypomethylation Hyperproliferative epithelium Early adenoma K-RAS Intermediate adenoma Initial ODE models of Wnt pathway focus on b-catenin’s role in regulating production of target genes (Lee et al, PLoS Biology, 2003) DCC? p53 Late adenoma Other alterations Carcinoma Metastasis APC mutation hyperproliferation and abnormal crypt morphology Simple model developed by Lee et al (2003) What level of detail? focuses on rolesSIGNALLING of Wnt and b-catenin in WNT regulating production of target genes … it is possible to generate many different models but it is often less obvious to know what level of detail to include Trends in Cell Biology 15, 2005 Current Biology 15, 2005 Science 303, 2004 WNT SIGNALLING ADHESION “Off” State β-catenin WNT-STIMULUS APC-complex DEGRADATION TRANSCRIPTION cell-cell adhesion complexes β-catenin degradation WNT SIGNALLING ADHESION “On” State WNT-STIMULUS ? β-catenin cell-cell adhesion complexes DEGRADATION TRANSCRIPTION β-catenin-TCF complexes Expression of target genes HYPOTHESIS-I (purely competitive scenario) APC complex β-catenin β-catenin TCF β-catenin Expression of target gene Y β-catenin cadherin Van Leeuwen et al (2007) J Theor Biol 247: 77-102 HYPOTHESIS-II (two molecular forms of β-catenin) TCF APC complex β-catenin β-catenin Wnt β-catenin β-catenin TCF β-catenin Expression of target gene Y β-catenin cadherin Van Leeuwen et al (2007) J Theor Biol 247: 77-102 MODEL EQUATIONS Wnt-dependent terms highlighted RESULTS 1 Effect of Wnt stimulation on gene expression Hypothesis II Hypothesis I (=32 hours) Build ODE model that combines both hypotheses Model Prediction: not possible to discriminate between hypotheses by measuring transcription levels RESULTS 2 Effect of Wnt stimulation on cell-cell adhesion Hypothesis I Model Prediction: may be possible to discriminate between hypotheses by measuring adhesion complexes Which model of Wnt signalling? Intracellular localisation of b-catenin Generating testable predictions Hypothesis I: pure competition between nucleus and membrane • At crypt base high levels of nuclear and membrane-bound bcatenin • Hypothesis II: bias towards nucleus • At crypt base, b-catenin concentrated in nucleus • Van Leeuwen et al, J theor Biol, 247 (2007) RESULTS 4 Effect of E-cadherin upregulation (external Wnt stimulus present) Model prediction: upregulating E-cadherin increases proportion of adhesion complexes but does not affect transcription (at steady state) (133 hours) MULTISCALE MODELLING OF COLORECTAL CANCER NORMAL CRYPT RENEWAL CYCLE 5 1. Proliferation of stem cells (bottom of crypt) 2. Progeny divides a few times (lower third of crypt) 3. Progeny starts migrating to the surface 3 4. Progeny differentiates (midcrypt region) 5. Senile cells are removed from the surface (midpoint between crypts) Above processes coordinated by Wnt signalling 4 2 1 Modelling CRC: MULTISCALE MODEL FRAMEWORK Multiscale Model Framework: MECHANICAL MODEL (CELL SCALE) © Gary Mirams, Nottingham Meineke et al. (2001) Cell Prolif 34: 253-266 Multiscale Model Framework Mechanical Model Fi(t) = ∑j kij [|ri(t) – rj(t)| – sij(t)] uij(t), ri(t + Δ t) = ri(t) + Fi(t) Δ t / ηi . where ηi = drag coefficient of cell i kij = spring constant between cells i and j Note: cell mechanics coupled to subcellular dynamics through drag and spring coefficients Meineke et al (2001) van Leeuwen et al (2009) Random cell-cycle time assigned to each daughter cell after division Cell cycle time determined by ODE (Wnt-dependent) cell-cycle model Differentiation occurs after fixed number of transit-cell generations Differentiation determined by extracellular Wnt gradient Cells simply “walk off” the 2D surface “Proper cell death” Movement determined by spring forces only Movement determined by spring forces and surface penalty function Normal cells only Normal and mutant cells HEALTHY CRYPTS: ARE THEY MONOCLONAL? Monoclonal conversion Are the stem cells pinned at the bottom of the crypt? Pinned stem cells (Meineke et al, 2001) Unpinned stem cells (van Leeuwen et al, 2009) Evidence for Monoclonal Crypts • Greaves et al (2006) PNAS 103: 714-719 • Taylor et al (2003) J Clin Invest 112: 1351-1360 Role of of geometry: Cylindrical vs projection Colorectal crypts Monoclonal conversion Osborne et al (in prep) EARLY CRC (LOSS OF APC): TOP-DOWN vs BOTTOM-UP? EXPANSION OF MUTANT CELLS (LOSS OF APC ) • Wnt gradient along crypt axis • Mutant cells identical to normals, except Wnt pathway always activated • Mutant cells washed out EXPANSION OF MUTANT CELLS (LOSS OF APC ) • Mutant cells: Wnt on & stronger cellsubstrate drag • Mutant cells persist • Consistent with ‘bottom-up’ theory Invasion of mutant cells: ‘Bottom-Up’ or ‘Top-Down’? • Mutant cells: Wnt on & stronger drag • Mutant cells persist & migrate downwards • Supports ‘topdown’ invasion Invasion of mutant cells: ‘Bottom-Up’ or ‘Top-Down’? Note: many simulations to determine probability that mutant cells persist CONTINUUM MODELLING Continuum Model Key parameters: D – Relative viscosity k – Gross proliferation λ1 – Wnt dependence • 0 < D < 1 mutant cells more sticky or adherent than normal cells • mutant cells proliferate independent of Wnt 44 Continuum model results D=1.0 D=0.2 45 Continuum model results Note: More timeconsuming to generate equivalent figures for cell-based models Note: Difficult to determine whether crypt monoclonal using continuum model and to account for subcellular 46 effects CONCLUSIONS CONCLUSIONS AND DISCUSSION • Wnt signalling: subcellular level • Competing roles of b-catenin in transcription and adhesion • Multiscale modelling of colonic crypts • Predict conditions under which crypts become monoclonal • Establish conditions under which ‘top-down’ and ‘bottom-up’ invasion may occur • Use of multiscale modelling for • Hypothesis testing • Generating testable predictions INTERACTIONS WITH THE STROMA Basement membrane separates layer of proliferating epithelial cells from tissue stroma Sara-Jane Dunn Multiscale Modelling: Future Work Absorptive columnar and mucinous cells Crypt fission • Model validation (in vitro, in vivo) • Distinguish different cell types • Crypt fission Polyp formation ACKNOWLEDGEMENTS: Oxford Nottingham Sara-Jane Dunn Alex Fletcher Oliver Jensen David Gavaghan John King Matthew Johnston Ingeborg van Leeuwen Sophie Kershaw Gary Mirams Philip Maini Alex Walter Philip Murray James Osborne Pras Pathmanathan Joe Pitt-Francis Jonathan Whiteley Dimensionless Equations