* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein mteabolism
Artificial gene synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Catalytic triad wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Lipid signaling wikipedia , lookup
Human digestive system wikipedia , lookup
Point mutation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Citric acid cycle wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Biochemistry wikipedia , lookup
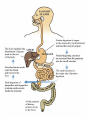
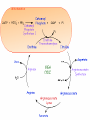
Protein metabolism Protein digestion: A) In stomach: passage of food into stomach stimulates gastric mucosa to secret a polypeptide hormone called: Gastrin which has the following actions: 1- stimulate the chief cells of gastric mucosa to secret the inactive zymogen “pepsinogen” 2- stimulates the parietal cells of gastric mucosa to secret HCl which activates pepsinogen into pepsin which activates more pepsinogen”autoactivation” Pepsin is an endopeptidase, partially hydrolyse the ingested proteins into polypeptides. HCl Pepsin Pepsinogen Auto activation pepsin Pepsinogen Pepsin B) In small intestine (Action of pancreatic enzymes): Pancreas secret several proenzymes into duodenum. The release and activation of pancreatic zymogens is mediated by the secretion of cholecystokinine and secretin (GIT hormones). Activation of pancreatic zymogens: The pancreatic zymogens are: trypsinogen, chymotrypsinogen and pro-carboxypeptidases (A and B). Enteropeptidase (formerly called enterokinase) converts trypsinogen into active trypsin which then activates more trypsinogen and the other proenzymes i.e activates chymotrypsinogen into chymotrypsin and pro-carboxypeptidase into carboxypeptidase. These enzymes hydrolyze polypeptides into oligopeptides. enteropeptidase Trypsinogen trypsin trypsin Trypsinogen trypsin Chymotrypsinogen trypsin chymotrypsin Other enzymes secreted into intestinal lumen are: 1- Amino peptidase: which hydrolyse Nterminal of amino acid to give free amino acids, dipeptides and tripeptides. 2-Dipeptidases and Tripeptidases: Hydrolyze dipeptides and tripeptides into free amino acids. So the final product of digestion is amino acids. NB: Carboxypeptiases and aminopeptidase are called exopeptidases as they hydrolyze protein at C-terminus and N-terminus, respectively. Pepsin, trypsin and chymotrypsin are endopeptidases Amino acids are absorbed by the intestinal mucosa and transported via blood stream. Once enter blood, amino acids rapidly enter cells. Liver and kidney take up the largest portion. Amino acid degradation Most of absorbed dietary amino acids are catabolized by 2 subsequent steps: 1- Removal of α-amino group: α-amino group is removed in the form of ammonia (NH3). This occurs in most amino acids by transamination followed by oxidative deamination of the resulting glutamate. 2- Breakdown of carbon skeleton. Removal of α-amino group: Transamination: is the transfer of α-amino group from α-amino acid to α-keto acid to yield α-keto acid of the original amino acid and a new amino acid. The enzymes that catalyze transamination are called transaminases or Aminotransferases. which need coenzyme pyridoxal phosphate (PLP) General reaction is: In most transamination reactions, the α-keto acid is α-ketoglutarate. The most common examples on transaminases are: 1- Alanine Aminotransferase (ALT) or called Glutamate-Pyruvate Transaminase (GPT): ALT 2- Aspartate Aminotransferase (AST) or called: Glutamate Oxaloacetate Transaminase (GOT). AST Clinical significance of aminotransferases: Aminotransferases are normally intracellular enzymes, and found only in low levels in plasma. The presence of elevated plasma levels of aminotransferases indcates damage of cells rich in these enzymes. e.g. ALT and AST are present in liver, so their elevation in blood indicate liver cell damage such as in hepatitis, toxic injury, cirrhosiss,…… Glutamate produced from transamination is oxidatively deaminated by the enzyme glutamate dehydrogenase yielding ammonia and regenerating α-ketoglutarate that is used in additional transamination reactions. The produced ammonia is toxic to CNS (neurotoxic) and it must be removed. Disposal routes of NH3 OR Removal of ammonia: 1- excreted with urine. 2- react with glutamate yielding glutamine by the enzyme glutamine synthetase this reaction occurs primarily in brain, muscles and liver. This reaction is the main disposal route of ammonia in brain 3- Used in formation of urea in liver. It is the most important disposal route of ammonia. Urea cycle or urea synthesis Site: urea is synthesized in the liver by 5 enzymes. Steps : see handout for detailed steps cytoplasm Notes on urea cycle: 1- Urea cycle involves 5 steps The first two reactions occur in Mitochondria while the steps 3,4 and 5 occur in Cytoplasm). 2- The rate limiting step in the cycle is the first reaction which is the formation of carbamoyl phosphate from CO2 and NH3 in the presence of carbamoyl phosphate synthetase I (CPSI) which is the rate limiting enzyme in the synthesis. 3-CPSI absolutely depends on the presence of N-acetyl glutamate which act as allosteric activator for the enzyme. 4- Ornithine and citrullin are two basic amino acids used in urea synthesis, but not enter in protein synthesis. What are the sources of N- and C atoms in urea? Fate of urea: 1- diffuse from liver, transported in blood to kidney and excreted with urine 2- a portion diffuse from blood to intestine and cleaved by bacterial urease into ammonia and CO2. Ammonia passes with stool. Hyperammonemia: 1- Genetic (hereditary hyperammonemia): Due to deficiency of one of the 5 enzymes of urea cycle (particularly CPSI) leading to increased level of ammonia during the first week of birth leading to mental retardation Precaution: limiting protein in diet 2) Acquired hyperammonemia - Is most commonly due to liver disease, especially in last stage where the capacity of live to synthesize urea is decreased Symptoms of hyperammonemia: Neurotoxic effects on CNS leading to: - Tremors , slurring of speech, blurring of vision, behavioral changes and cerebral edema -At very high levels: coma and death - A hyperammonemic coma occurs when serum ammonia concentrations are greater than 300 μmol/L and is a medical emergency requiring immediate treatment designed to prevent irreversible brain damage.