* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amino acid catabolism I
Metabolic network modelling wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Magnesium transporter wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Proteolysis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Citric acid cycle wikipedia , lookup
Genetic code wikipedia , lookup
Calciseptine wikipedia , lookup
Biochemistry wikipedia , lookup
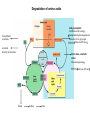
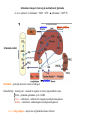
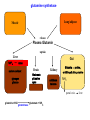
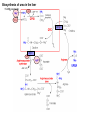
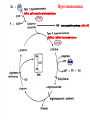
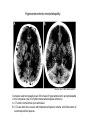
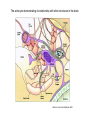
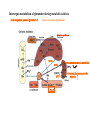
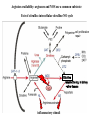
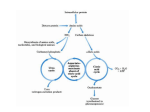
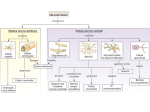
Protein synthesis break down Amino acids de novo Oxidation synthesis Muscle Transamination Amino acids Blood Schematic protein turnover and metabolic fates Alanine Glutamine Glutamate Muscle will not improve with protein feeding alone! Fed-state gains and fasted state losses in muscle protein balance Skeletal muscle mass is maintained by normal protein feeding. Feeding refreshes muscle protein to improve muscle function, permitting more physical activity. Overfeeding of protein increases insulin resistancemuscle proteolysis Fed state gains are enhanced, fasted state losses are less Improvement in immune function stimulation of protein synthesis Degradation of amino acids rich protein diet: Catabolised for energy, postprandial gluconeogenesis stored as liver glycogen PEPCK Glucose-6P-ase biosynthetic reactions excreted directly in the urine AMP purine nucleotide cycle Starvation, catabolic states: Gluconeogenesis IMP PEPCK Glucose-6P-ase (liver) blood kidney urine Amino acid catabolism I: Fate of the nitrogen Central role of glutamate in nitrogen metabolism Ser Thr amino acid a-keto-glutarate + oxidative deamination NH4 transamination keto acid His Asp Glutamate Gln - Amino group from the majority of amino acids is collected by glutamate (by transamination) in the hepatocytes. - Liberation of the amino group in the formation of NH+4 by GDH. L-glutamate dehydrogenase reaction Glutamate in amino acid synthesis, degradation and interconversion glutamate glutamine NH4+ other amino acids intestinal bacteria Allosteric regulation of glutamate dehydrogenase Catabolism of L-amino acids Transaminases (aminotransferases) GOT Glutamate + oxaloacetate (OAA) <---> a-ketoglutarate + aspartate ASAT GPT Glutamate + pyruvate <---> a-ketoglutarate + alanin ALAT Coupled transamination reaction PRP Pyridoxal phosphate (PRP) and PRP in aldimine linkage to the lysine residue of the transaminase (Schiff-base) Different forms of pyridoxal phosphate during a transamination reaction R1- R2 E R1 R2pyridoxamine phosphate Specific pathways for the deamination of amino acids (minor routes) Serine dehydratase Metabolism of serine for gluconeogenesis cystein desulphhydrase D-amino oxidases (FAD), L-amino acid oxidase (FMN) Transport of ammonia Concentration of ammonia in the systemic blood is very low (25-50μmol/L), toxic to the brain. Transport: glutamine and alanine (muscle) glutamine ( brain) Glutamine: non-toxic carrier 0.5-0.8mM in arterial plasma, 20-25% of circulating free amino acids precursor for synthesis of many nitrogen containing compounds metabolic fuel for rapidly dividing cells generates glutamate and GABA in the brain Glutamine transport , interorgan metabolism of glutamine de novo synthesis: L-Glutamate + NH4+ + ATP L-Glutamine + ADP+ Pi compartmentalised glutaminase glutamine synthetase Glutamine in diet low glutaminase Glutamine - principle non-toxic carrier of nitrogen Intracellularly – muscle pool – released in response to stress, hypercatabolic states brain – glutamine-glutamate cycle- GABA liver – catabolised - substrate for ureagenesis and gluconeogenesis kidney – catabolised - ammoniagenesis and gluconeogenesis muscle, lung, adipose - major sites of glutamine release to blood glutamine synthetase Muscle Lung/adipose Muscle release Plasma Glutamin uptake Liver NH+ 4 Gut urea carbon sceleton: glycogen glucose Brain Kidney Glutamateglutamine cycle acid-base balance Glutamin ornithine,citrulline, alanine NH3 portal vein glutamine+H2O glutaminase glutamate + NH 3 proline, liver The liver receives both amino acids and ammonia from circulation Scource of ammonia in different tissues: 1. degradation of amino acids transdeamination (transamination+GDH) minor patways 2. deamination of other compounds N-containing side chains of nucleotides neurotransmitters 3. ammonia production in the large intestine by bacteria portal vein, direct transport of ammonia. Urea cycle Function: 1. prevents ammonia levels from rising too high when large amounts of amino acids are catabolized 2. urea cycle enzymes: extrahepatic arginine synthesis Biosynthesis of urea in the liver 55-100g protein/day ORNT1 ORNT1 The liver receive both ammonia and amino acids from the circulation GDH and major aminotransferases catalyze reactions close to equilibrium Quantitative aspects of nitrogen incorporation, regulation? Regulation of the urea cycle 1. Short term: NAG an allosteric regulator of CPSI and glutaminase activity increased amino acid catabolism + increase in NAG increased flux with constant ammonia concentration increase in glutamate, more NAG glutaminase Arginine + mitochondria 2. Long term: high protein diet: transcriptional regulation. Hepatic glycogen syntesis Caloric restriction: increased protein catabolism – CPSI induction (cAMP responsive element), glucose need. ORNT1 - increased transcription. Hyperammonemias deffect: carbamoyl phosphate synthetase CPSD CP cytosol, pyrimidine synthesis, orotic acid deffect: ornithine transcarbamoylase OTCD NH4+ Inherited urea cycle diseases (+liver failure) Hyperammonemia Brain edema, convulsions, coma Having no urea cycle, brain relies on glutamine synthetase for the removal of exes ammonia NH3 Change in astrocyte morphology: cell swelling astrocytosis acute hyperammonemia chronic hyperammonemia Changes in expression of glutamate transporters in astrocytes. Scriver et.al.The metabolic and Molecular Bases of Inherited Deseases,2001 Hyperammoniemic encephalopathy Brusilov: Rev. in Mol. Medicine,2002 Computer axial tomography scan of the head of hyperammonemic encephalopathy in the composite case of ornythine transcarbamoylase deficiency. A. CT within normal limits upon admission B. CT scan after tonic seizure with bilateral hemispheric edema with effacement of cerebrospinal fluid spaces. The actrocyte demonstrating its relationship with other structures in the brain Brusilov: Rev.in Mol. Medicine,2002 The glutamate synapse, effect of NH3 on the the Glutamate-glutamine cycle intracellular Glu depletion glutaminase NH3 glutamine synthetase extracellular accumulation Ca2+ NO Brain injury Felipo et.al.:Progress in Neurobiology,2002 Treatment: - limited nitrogen diet - arginine becomes an essential amino acid - detoxification reactions as alternatives to the urea cycle, ATP dependent Hepatic metabolism of glutamine, zonal distribution of glutaminase and glutamine synthetase bulk remaining detoxify glutaminase glutamine synthetase high affinity high capacity low affinity spare aminonitrogen in starvation at metabolic acidosis: net producer of glutamine Sequential synthesis of urea and glutamine – efficient to ensure systemic/nontoxic level of ammonia Ammonium ion - feed-forward activator of synthesis of glutamate and N-acetyl glutamate Hepatic synthesis of glutamine – acid-base balance. Decrease pH – activation of glutamine synthetase –sparing of glutamine Interorgan metabolism of glutamine during metabolic acidosis Acut response: plasma glutamine Renal extraction of glutamine glutamine synthetase release uptake pH incresased ammonia excretion increased gluconeogenesis PEPCK The urea cycle – part of the metabolism centered around L-arginine L-arginine is semiessential amino acid, synthesized in collaboration. The intestinal – renal axis. Arg Bioavailability of arginine is complex 1.Exogenous supply 2.Endogenous release 3.Arginine resynthesis 4.Arginine catabolism, arginase 5.Arginine transport CAT-1 AS, AL EC, nerve cells, macrophages urea arginase Arg CAT-1 Arg NO+citrulline circulation Insufficient Arg: strict carnivors small bowel, kidney disease conditions with elevated amino acid catabolism: inflamation, sepsis, recovery. Arginine availability: arginases and NOS use a common substrate Fate of citrullin: intercellular citrulline-NO cycle - cell proliferation repair Citrulline recycled to Arg, in kidney +other tissues inflammatory stimuli Arginine is the largest scource for NO production NO,(EDRF): labile, common gas NO-cGMP-mediated effects: smooth muscle cell relaxation in EC: cGMP-prostacyclin mediated decrease in platelet aggregation decrease in leukocyte adhesion and migration NO functionality – vascular health/vasculopathy - production of NO – depends on NOS activity “Arginine paradox” Km for eNOS: 1.4-2.9 μmol/L Intracellular L-arginine: 0.5-2mmmol/L eNOS should be saturated with substrate Despite high cellular arginine, and low Km of eNOS: arginine, citrulline supplementation “in vivo” improves NO function: increased vasodilation decreased leukocyte adhesion decreased platelet adhesion Possible reasons: altered arginine transport increased arginase activity compartmentalisation of arginine Supplementation: Arg: low bioavailability, increased arginase Cit: Arg synthesis, increased NO levels Gln: major vehicle of transport, Glu-gluthatione reduction of oxidative stress Gly: restores NO balance at increased nutrient demands Meth, Homocys: increased cardiovascular risk Lys: decreases Arg transport