* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 6 POWERPOINT here
Multi-state modeling of biomolecules wikipedia , lookup
Gene regulatory network wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Signal transduction wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Citric acid cycle wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biochemical cascade wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
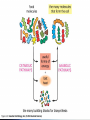
Energy management within cells Lecture 6 Controlled Pathways The various compartments of the cell (- what are they?) are populated with a very large number of chemical reagents, products, and enzymes. How does the cell control them all? Each step in this pathway is regulated by specific enzymes this is one mechanism which allows multiple reactions to occur in a common environment. Pathways A complex pathway can further be regulated by a number of different feedback mechanisms - both up regulation and down regulation, feedback inhibition and feedback initiation, and other more complex interactions. -Watch Multimedia Biochemical Pathways FileName: Bio10.swf The biosynthetic pathway for the two amino acids E and H is shown schematically below. You are able to show that E inhibits enzyme V, and H inhibits enzyme X. Enzyme T is most likely to be subject to feedback inhibition by __________________ alone. (a) (b) (c) (d) (e) A B C E H An average cell has both general reactions which it needs to perform to sustain life, as well as specialized ones that make that cell type unique, i.e. pancreatic cell. The general reactions are called housekeeping reactions These can be many in number and their interactions are pretty complex… Anabolic & Catabolic Regardless of the complexity they are of two types ANABOLIC CATABOLIC … Enzymes Vast majority are P’s (however, some RNA) Increase the rate of virtually ALL chemical reactions - fact: A reaction that takes just milliseconds in the presence of an enzyme would take millions of years without (some increase the rate by as much as 1 x 1018 fold!!!) Enzyme pool selectively determines which reactions shall take place inside a cell & when Enzymes… Catalysts - Biological Catalysts 2 Main Properties 1. Increase rate without change to enzyme 2. Do not alter chemical equilibrium Just speed things along by bringing molecules together and reducing the activation energy of the reactions too. Random Motion The meeting of substrates and substrates and enzymes is random. The meeting is driven by the thermal energy of the molecules at these temperatures Quicktime movie (rmotion.mov) .… Activation Energy An important concept that you have to learn Enzymes mechanisms Enzymes are specific AA’s from different parts of the P’ come together to form the active site (binding pocket) ‘Lock-and-key’ model - exact fit Induced fit model - alteration of the substrate by the binding process Enzyme kinetics Initial binding is ionic Subsequent interactions may involve covalent exchanges Atomic distances involved Prosthetic groups - small molecules that participate in catalysis - metal ions Coenzymes - small molecules that enhance rates - organic molecules - Biotin Enzyme regulation Activity can be modulated - controlled to suit the needs of the cell Feedback inhibition - product inhibits more product formation Allosteric regulation - ‘other - site’ - molecules which bind to the enzymes to alter its physical properties Phosphorylation - adding of phosphate groups to P’ to regulate activity - serine, threonine, or tyrosine AA’s - : + or - Metabolic Energy Cells need energy to function, grow and multiply A large portion of the cells resources are spent on obtaining energy Most reactions utilize energy Gibbs FREE ENERGY = ∆G - release of energy is -∆G ATP = ∆G of @ -12kcal/mol - releases energy on hydrolysis Glycolysis (covered in greater detail later in this course) Breakdown of glucose for energy to Pyruvate ∆G = -686 kcal/mol Nearly every cell performs glycolysis No oxygen required - anaerobic reaction Location - cytoplasm Does this same reaction occur in bacteria? Where does this same reaction occur in bacteria? Acetyl CoA (covered in greater detail later in this course) Acetyl coenzyme A Intermediary in metabolism Forms when Coenzyme A reacts with pyruvate Eukaryotes - mitrochondria Citric acid cycle (covered in greater detail later in this course) Krebs cycle Oxidative metabolism Mitrochondria Photosynthesis (covered in greater detail later in this course) Sunlight is the ultimate source of energy Plants and bacteria produce carbohydrates through photosynthesis Chlorophylls - photosynthetic pigments Stay Current Please Read chapter 2 fully & visit the website.