* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the Four Stages of Biochemical Energy Production
Butyric acid wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
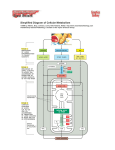
Stages 3 & 4 • These stages are the same for all types of food = “common metabolic pathway” • They occur inside the mitochondria – Citric Acid (Kreb’s) Cycle – Electron Transport Chain & Oxidative Phosphorylation The Citric Acid Cycle • Maynard G. Krebs • Hans Adolf Krebs Citric acid cycle: Oxidation of the products of food breakdown releases energy which is carried by NADH and FADH2 molecules to be turned into ATP later. 1 ATP, 3 NADH & 1 FADH2 form with each cycle. Citric acid cycle, Krebs cycle, or Tricarboxylic acid cycle: the main types of reactions found at each step. Citric acid cycle – For every glucose, two acetyl groups enter the citric acid cycle (Krebs cycle) • Each two-carbon acetyl group combines with a fourcarbon compound • Two CO2 molecules are removed (why is this important?) • Energy captured as 1 ATP, 3 NADH, and 1 FADH2 form from each acetyl group Dehydration synthesis or condensation reaction Oxaloacetate 4 C molecule: Acetyl CoA transfers its acetyl group to the #2 carbonyl carbon via the methyl end. This forms a 6 C citrate molecule. First dehydration then hydration. Why? Aconitase enzyme catalyzes both reactions Citrate: Note that it is a tertiary alcohol which is not oxidizable. Isomer is a 2o alcohol (isocitrate) that can be oxidized First of 4 Redox reactions, followed by decarboxylation. NAD+ is the oxidizing agent. Isocitrate dehydrogenase is the catalyst Alpha ketoglutarate (a ketone) is formed when Isocitrate is oxidized, leading to the reduction of NAD+ to NADH, & also decarboxylated (first CO2 formed) Redox reaction #2: Oxidation of a-ketoglutarate into 4 C Succinyl CoA (also a ketone) again more CO2 and NADH are formed Three enzymes used as a group: a-ketoglutarate dehydrogenase complex. H atom must be removed from sulfhydryl group for coenzyme A to form a high energy thioester bond. Thioester bond cleavage (woo!) in Succinyl CoA & Phosphorylation of Guanosine diphosphate (GDP) Succinate formation: The thioester bond in succinyl CoA is hydrolyzed forming succinate, with generation of GTP (which in turn forms ATP) Steps 6 - 8 involve a series of functional group changes: oxidation of an alkane (dehydrogenation) to form an alkene. Then hydration to form a 2o alcohol, & finally oxidation (dehydrogenation) to form a ketone. 3rd Redox reaction: Succinate oxidized by FAD forms fumarate (-2 H atoms). Fumarate is an alkene with a trans double bond. The cis form is called maleate and is toxic. Fumarate undergoes hydration catalyzed by fumarase which catalyzes addition of water to the double bond. Only L-Malate isomer is formed This makes a secondary alcohol. What reaction do you think will happen next? Oxidation of L-Malate to regenerate 4 C Oxaloacetate molecule. Cycle begins again! NAD+ is again the oxidizing agent, So more reduced NADH is formed. All of these reduced carrier molecules go on to the last step: the electron transport chain. Review: can you… • Explain what is meant by the term “Metabolism“ • Describe what organelles are and the structure of a mitochondria. • Compare and contrast the important intermediate compounds in metabolic pathways. • Explain what “High-Energy” means using phosphate compounds as examples • List the 4 steps of biochemical energy production • Give a detailed sequence of the reactions involved in the Citric Acid Cycle Electron transport chain Series of electron carriers Each carrier exists in oxidized or reduced form High energy electrons pass down the electron transport chain in a series of redox reactions These reactions are coupled with ATP synthesis (oxidative phosphorylation). They lose energy as they pass along the chain Oxidative (chemiosmotic) phosphorylation Within the Mitochondria of cells: Chemical and electrical concentration gradients supply energy to enzymatically produce ATP from ADP & Pi 6 NADH & 2 FADH2 transport glucose energy (e- & H+ ions) to E.T.C. A series of special protein trans-membrane molecules, cytochrome molecules, a special lipid (ubiquinone) are all embedded in the inner membrane of the mitochondria. The electrons delivered by the carrier molecules (NADH and FADH2) begin at high energy levels. Electron transport chain (ETC) inner membrane is up to 75% protein by mass - allows only O2, CO2 and H20 to freely pass Membrane proteins composed of redox molecules. Fixed enzyme Complex #1 (entrance of NADH): Step #1: FMN (Flavin mononucleotide) and Step #2: FeSP (iron sulphur protein) Step #3: Mobile enzyme: CoQ (Coenzyme Q) (entrance of FADH2) Fixed enzyme Complex #2: Step #4: cyt b (cytochrome b) and Step #5: cyt c1 (cytochrome C1) Step #6: Mobile enzyme: cyt c (cytochrome C) Fixed enzyme Complex #3: Step #7: cyt a (cytochrome a) and Step #8: cyt a3 (cytochrome a3) ETC animation