* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Single-Replacement Reactions
Radical (chemistry) wikipedia , lookup
Photoredox catalysis wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Acid–base reaction wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Safety data sheet wikipedia , lookup
Fine chemical wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Chemical Corps wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Marcus theory wikipedia , lookup
Isotopic labeling wikipedia , lookup
Asymmetric induction wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Chemical plant wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
History of chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemical industry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Rate equation wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
George S. Hammond wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Transition state theory wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
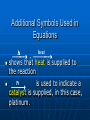
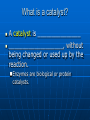
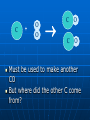
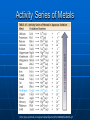
Chemical Equations and Reactions Chapter 8 General Chemistry Chemical Reactions Basics Chemical Reactions A chemical reactions are ______________________. Recall: chemical changes are when one or more substances are changed into different substances • Examples: wood burning, metal rusting Indications of a Chemical Reaction Certain easily observed changes indicate that a chemical reaction has occurred: _______________by heat and/or light color change production of _________ production of __________ A solid that is produced as a result of a chemical reaction in solution and that separates from the solution is known as a precipitate Law of Conservation of Matter Chemical reactions obey the Law of Conservation of Matter in all chemical and physical changes, matter is neither created or destroyed __________________________________ __________________________________ Chemical Equations A chemical equation represents, with symbols and formulas, the identities and the amounts of the reactants and products in a chemical reaction. Has two parts: • Reactants: __________________________ • Products: ____________________________ The reactants turn into the products 4 Fe + 3 O2 2 Fe2O3 Reactants products Ways to Express a Chemical Reaction The way atoms are joined is changed in a chemical reaction. Can be described several ways: 1. In a sentence Copper reacts with chlorine to form copper (II) chloride. 2. In a word equation Copper + chlorine copper (II) chloride 3. In formula equation: Cu + Cl2 CuCl2 Symbols Used in Chemical Equations Additional Symbols Used in Equations heat , shows that heat is supplied to the reaction Pt is used to indicate a catalyst is supplied, in this case, platinum. What is a catalyst? A catalyst is _______________ ___________________, without being changed or used up by the reaction. Enzymes are biological or protein catalysts. Study Buddy Review What are four things that indicate a chemical reaction has taken place? What is the Law of Conservation of mass? What does the symbol (l) mean? What does (aq) mean? Balancing Chemical Equations Skeleton and Balanced Equations _____________________do not indicate how many of each element/compound _______________________the number of atoms of each element is the same on both sides of the reaction. Numbers in Balanced Equations Use coefficients to balance equation Coefficients: _______________________ ______________which represents number of units of that compound Subscript: small whole number placed in chemical formula to represent number of atoms of an element in a compound 4 Fe + 3 O2 Coefficient 2 Fe2O3 subscript Balanced Equation A balanced equation has the _____________________ of each element on both sides of the equation Atoms can’t be created or destroyed All the atoms at the beginning must appear in the end C + O O C O C + O2 CO We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) C + O O C O C O Must be used to make another CO But where did the other C come from? C + C O O C O C O Must have started with two C 2 C + O2 2 CO Rules for balancing: Assemble, write the correct formulas for all the reactants and products Count the number of atoms of each type of element appearing on both sides Balance the atoms of an element one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. Never change a subscript to balance an equation. • If you change the formula you are describing a different reaction. • H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula • 2 NaCl is okay, Na2Cl is not. Balancing Equations Examples H2 (g) + O2 (g) Zn + Pb (NO3)2 + HCl H2O (l) H2 + K2S ZnCl2 PbS + KNO3 Word Equation Examples Write a balanced chemical equation for the following: Aqueous solutions of sulfuric acid and sodium hydroxide react to form aqueous sodium sulfate and water. Five General Types of Chemical Reactions Five General Types of Chemical Reactions 1. 2. 3. 4. 5. Synthesis (Composition) ________________________ Single-Replacement ________________________ Combustion By knowing the type of reaction that is occurring, you can predict the products that will be formed. General Types of Chemical Reactions Combination: A + B AB Decomposition: AB A + B Single Replacement : AX +B A + BX Double Replacement: AX + BY AY + BX Combustion: CxHy+ O2 CO2 + H2O Synthesis (Combination) General form: A + B AB ____________________________ A, B = elements or compounds AB = compound consisting of A and B This is the only type of chemical reaction in which there is a single product formed. This single product is always more complex than the reactants. Examples of Synthesis Reactions a. calcium + oxygen yields calcium oxide 2Ca + O2 2CaO b. carbon dioxide + water yields carbonic acid CO2 + H2O H2CO3 Notice: All equations show two (or more) reactants, but only one product. http://www.ric.edu/ptiskus/reactions/Index.htm Decomposition Reactions (Decomposing = breaking down into smaller parts; microorganisms) _______________________________ ______________________________The opposite of direct combination/synthesis. You can identify this reaction because there is only one reactant. General form: AB A + B AB = compound A, B = elements or simpler compounds Examples of Decomposition Reactions Water yields hydrogen and oxygen 2H2O 2H2 + O2 marble (rock) yields (with heating) calcium oxide and carbon dioxide CaCO3 CaO + CO2 Notice: single compound decomposes into two (or more) products. http://www.ric.edu/ptiskus/reactions/Index.htm Single-Replacement Reactions _____________________________ _____________________________ General Form: A + BX AX + B AX, BX = ionic compounds A, B = elements X = ion that switches partners Examples of Single-Replacement Reactions Magnesium metal and copper (II) sulfate Mg + CuSO4 MgSO4 + Cu Iron metal and copper (II) sulfate) Fe + CuSO4 FeSO4 + Cu www.ric.edu/ptiskus/reactions/Index. htm Double-Replacement Reactions _______________________________ _______________________________ Look for: 2 compounds as reactants and 2 compounds as products. Reactants are usually ionic compounds. Specific example: neutralization reactions between acid and base General form: AX + BY AY + BX (Notice: X and Y “change partners”) Examples of Double Replacement Reactions Calcium carbonate and hydrochloric acid yield calcium chloride and carbonic acid. CaCO3 + 2HCl CaCl2 + H2CO3 www.ric.edu/ptiskus/reactions/Index. htm Rules of Double-Displacement Reactions Reactants must be dissolved in water (releasing the ions). Will occur if: 1. ______________________________ 2. ______________________________ 3. _____________________ Combustion Reactions Reactants: a fuel (usually a hydrocarbon) and oxygen. Products: water, carbon dioxide, and lots of energy! (heat, light) Examples: CH4 (methane) + 2O2 2H2O + CO2 C2H5OH (gasohol) + 3O2 3H2O + 2CO2 www.ric.edu/ptiskus/reactions/Index.htm Study Buddy Review Describe each of the following to your study buddy: • Synthesis (combination) • Decomposition • Single-Replacement • Double-Replacement • Combustion Activity Series Activity Series and Single Replacement Reactions Al + Cu + AlCl3 ??? CuCl2 ??? Only one of the following reactions will occur How do you know which one? Activity Series of Metals http://wps.prenhall.com/wps/media/objects/165/169060/tool0403.gif Activity Series Rules Elements at top of series are most active Elements at bottom of series are least active (coinage metals) _____________________________ _____________________________ Example: Al + CuCl2 will occur Cu + AlCl3 will NOT occur Activity Series Examples Using the activity series, predict whether each of the possible reactions will occur: • Cr + H2O • Pt + O2 • Cd + 2HBr References Dr. Stephen L. Cotton Charles Page High School Mrs. Lijek