Microporous polymer beads for chemical
... 2.1 Chemical warfare agents 2.1.1 History of chemical warfare agents The use of chemical warfare agents (CWAs) can be documented throughout the Middle Ages and Renaissance, but the modern use of chemical warfare began in the early twentieth century during World War I (WWI) when German military force ...
... 2.1 Chemical warfare agents 2.1.1 History of chemical warfare agents The use of chemical warfare agents (CWAs) can be documented throughout the Middle Ages and Renaissance, but the modern use of chemical warfare began in the early twentieth century during World War I (WWI) when German military force ...
Selective 15N labeling and direct observation by NMR
... heme Fe, often in low-spin states. By contrast, the nonheme Fe of Fe-SOD is high-spin in both oxidation states. However, the Gln implicated in determining the metal ion specificity of Fe-SOD is one of only three glutamines in the molecule, so selective labeling methods offer immediate access to the ...
... heme Fe, often in low-spin states. By contrast, the nonheme Fe of Fe-SOD is high-spin in both oxidation states. However, the Gln implicated in determining the metal ion specificity of Fe-SOD is one of only three glutamines in the molecule, so selective labeling methods offer immediate access to the ...
EVS - RSC - Developments in Microwave Chemistry
... • The research, which was conducted to study intellectual property relating to microwave chemistry, is primarily based on patent publications in the public domain. Patent publications that have still not been published, containing information about recent developments in the field, could not be incl ...
... • The research, which was conducted to study intellectual property relating to microwave chemistry, is primarily based on patent publications in the public domain. Patent publications that have still not been published, containing information about recent developments in the field, could not be incl ...
Chemical Transport Model - Technical Description
... comprises general statements based on scientific research. The reader is advised and needs to be aware that such information may be incomplete or unable to be used in any specific situation. No reliance or actions must therefore be made on that information without seeking prior expert professional, ...
... comprises general statements based on scientific research. The reader is advised and needs to be aware that such information may be incomplete or unable to be used in any specific situation. No reliance or actions must therefore be made on that information without seeking prior expert professional, ...
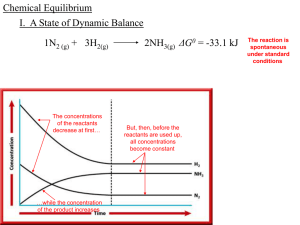
Chemical Equilibrium
... ability to affect the equilibrium. Chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. When we stress the equilibrium, the chemical reaction is no longer at equilibrium, and the reaction starts to move back toward equilib ...
... ability to affect the equilibrium. Chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. When we stress the equilibrium, the chemical reaction is no longer at equilibrium, and the reaction starts to move back toward equilib ...
88KB
... NMR Spectra of the Self-Cleaved Form of the Trans HDV Ribozyme. Initial 1H NMR characterization of the self-cleaved form of the ribozyme revealed a well-dispersed imino region of the spectrum with about 20 distinguishable peaks (Figure 1S, Supporting Information). No proton resonance in the 15 ppm r ...
... NMR Spectra of the Self-Cleaved Form of the Trans HDV Ribozyme. Initial 1H NMR characterization of the self-cleaved form of the ribozyme revealed a well-dispersed imino region of the spectrum with about 20 distinguishable peaks (Figure 1S, Supporting Information). No proton resonance in the 15 ppm r ...
Chemical Equilibrium - 2012 Book Archive
... This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to completion, that all the H2 and the I2 react to make HI. However, this is not the case. The reverse chemical reaction ...
... This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to completion, that all the H2 and the I2 react to make HI. However, this is not the case. The reverse chemical reaction ...
Management of Peroxide Forming Chemicals
... Peroxide-forming chemicals are compounds that may react with oxygen, even in low concentrations and at temperatures often not considered hazardous. Peroxidation is a hazard affecting primarily liquid peroxide formers and finely divided solids. The risk of peroxide formation exists when the compound ...
... Peroxide-forming chemicals are compounds that may react with oxygen, even in low concentrations and at temperatures often not considered hazardous. Peroxidation is a hazard affecting primarily liquid peroxide formers and finely divided solids. The risk of peroxide formation exists when the compound ...
Chemical Reactions - 2012 Book Archive
... chemical compound has a particular combination of atoms and that the ratios of the numbers of atoms of the elements present are usually small whole numbers. We also described the law of multiple proportions, which states that the ratios of the masses of elements that form a series of compounds are s ...
... chemical compound has a particular combination of atoms and that the ratios of the numbers of atoms of the elements present are usually small whole numbers. We also described the law of multiple proportions, which states that the ratios of the masses of elements that form a series of compounds are s ...
Modeling Multi-typed Structurally Viewed Chemicals with the UMLS
... structurally viewed CSTs, a chemical reaction may occur and produce an entirely new chemical. Such a chemical is called a conjugate. A conjugate chemical does not necessarily have all of the properties of its source chemicals, because some of the original structural components are expended in its cr ...
... structurally viewed CSTs, a chemical reaction may occur and produce an entirely new chemical. Such a chemical is called a conjugate. A conjugate chemical does not necessarily have all of the properties of its source chemicals, because some of the original structural components are expended in its cr ...
DEPARTMENT OF CHEMISTRY Requirements For Chemistry Major
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
Quantitative Comparison of the Hydrogen Bond
... Comparison of the H-Bond Network in the A-State and in the NatiVe State. The transition from the native state to the A-state leads to a very striking reshuffling of the H-bond network in ubiquitin, which can be followed in detail by the h3 JNC′ scalar correlations between H-bond donor and acceptor a ...
... Comparison of the H-Bond Network in the A-State and in the NatiVe State. The transition from the native state to the A-state leads to a very striking reshuffling of the H-bond network in ubiquitin, which can be followed in detail by the h3 JNC′ scalar correlations between H-bond donor and acceptor a ...
Chapter 5: Calculations and the Chemical Equation
... Evaluating Success of Synthesis One of the most important contribution of chemistry to other areas of sciences providing chemicals or raw materials for building various components of complex structures. Chemists come up with chemical reactions and optimize the conditions at which highest yield of th ...
... Evaluating Success of Synthesis One of the most important contribution of chemistry to other areas of sciences providing chemicals or raw materials for building various components of complex structures. Chemists come up with chemical reactions and optimize the conditions at which highest yield of th ...
Stoichiometry and the Mole
... But it also goes beyond carbon. Previously we defined atomic and molecular masses as the number of atomic mass units per atom or molecule. Now we can do so in terms of grams. The atomic mass of an element is the number of grams in 1 mol of atoms of that element, while the molecular mass of a compound ...
... But it also goes beyond carbon. Previously we defined atomic and molecular masses as the number of atomic mass units per atom or molecule. Now we can do so in terms of grams. The atomic mass of an element is the number of grams in 1 mol of atoms of that element, while the molecular mass of a compound ...
Subject Area Standard Area Organizing Category Grade Level
... CHEM.A.2.1.1: Describe the evolution of atomic theory leading to the current model of the atom based on the works of Dalton, Thomson, Rutherford, and Bohr. ...
... CHEM.A.2.1.1: Describe the evolution of atomic theory leading to the current model of the atom based on the works of Dalton, Thomson, Rutherford, and Bohr. ...
Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic
... With a 50 % Yield, How many moles of NH3 are produced from (a) 3 grams of H2 and ½ mole of N2? ½ mole = (½ mole)x(17 g/mole) grams of NH3 (b) 3 grams of H2 and 28 grams of N2? ...
... With a 50 % Yield, How many moles of NH3 are produced from (a) 3 grams of H2 and ½ mole of N2? ½ mole = (½ mole)x(17 g/mole) grams of NH3 (b) 3 grams of H2 and 28 grams of N2? ...
Stoichiometry and the Mole - 2012 Book Archive
... 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it wa ...
... 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it wa ...
Slide 1
... • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chloride and Keq. ...
... • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chloride and Keq. ...
U3 Student Workbook - The Connected Chemistry Curriculum
... Chemical and physical changes constantly happen both inside and around us. In the exploration of this unit, you will focus on the relationship between chemical changes and reactions. Chemists communicate with each other about their observations of chemical reactions using the formal names of differe ...
... Chemical and physical changes constantly happen both inside and around us. In the exploration of this unit, you will focus on the relationship between chemical changes and reactions. Chemists communicate with each other about their observations of chemical reactions using the formal names of differe ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
Chapter 8 - Chemical Equations and Reactions
... CH4(g) + Al(OH)3(s) (not balanced) • Balance Al atoms Al4C3(s) + H2O(l) CH4(g) + 4Al(OH)3(s) (partially balanced) Chapter menu ...
... CH4(g) + Al(OH)3(s) (not balanced) • Balance Al atoms Al4C3(s) + H2O(l) CH4(g) + 4Al(OH)3(s) (partially balanced) Chapter menu ...
Chapter 8 Notes
... CH4(g) + Al(OH)3(s) (not balanced) • Balance Al atoms Al4C3(s) + H2O(l) CH4(g) + 4Al(OH)3(s) (partially balanced) Chapter menu ...
... CH4(g) + Al(OH)3(s) (not balanced) • Balance Al atoms Al4C3(s) + H2O(l) CH4(g) + 4Al(OH)3(s) (partially balanced) Chapter menu ...
Chemical Vapor Deposition (CVD)
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
Chemistry: Percent Yield
... a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv Calculate simple mole-mole stoichiometry problems, given a balanced equation 3.3vi Determine the mass of a give ...
... a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv Calculate simple mole-mole stoichiometry problems, given a balanced equation 3.3vi Determine the mass of a give ...
Chemical weapon

A chemical weapon (CW) is a munition that uses chemicals formulated to inflict death or harm on human beings. The Organisation for the Prohibition of Chemical Weapons (OPCW) states: The term chemical weapon may also be applied to any toxic chemical or its precursor that can cause death, injury, temporary incapacitation or sensory irritation through its chemical action. Munitions or other delivery devices designed to deliver chemical weapons, whether filled or unfilled, are also considered weapons themselves.They are classified as weapons of mass destruction (WMDs), though they are distinct from nuclear weapons, biological weapons (diseases), and radiological weapons (which use radioactive decay of elements). All may be used in warfare known by the military acronym NBC, for nuclear, biological, and chemical warfare. Weapons of mass destruction are distinct from conventional weapons, which are primarily effective due to their explosive, kinetic, or incendiary potential. Chemical weapons can be widely dispersed in gas, liquid and solid forms, and may easily afflict others than the intended targets. Nerve gas, tear gas and pepper spray are three modern examples.Lethal, unitary, chemical agents and munitions are extremely volatile and they constitute a class of hazardous chemical weapons that are now being stockpiled by many nations. (Unitary agents are effective on their own and require no mixing with other agents.) The most dangerous of these are nerve agents GA, GB, GD, and VX, and vesicant (blister) agents which are formulations of sulfur mustard such as H, HT, and HD. All are liquids at normal room temperature, but become gaseous when released. Widely used during the First World War, the effects of so-called mustard gas, phosgene gas and others caused lung searing, blindness, death and maiming.Pepper spray is of common use today. It is potentially lethal. There are no recent records of pepper spray being used in war, despite the fact that it inflicts fewer injuries and side-effects compared with impact and explosive weapons.Under the Chemical Weapons Convention (1993), there is a legally binding, world-wide ban on the production, stockpiling, and use of chemical weapons and their precursors. Notwithstanding, large stockpiles thereof continue to exist, usually justified as only a precaution against putative use by an aggressor.