EVS - RSC - Developments in Microwave Chemistry
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
Chemical Reactions - 2012 Book Archive
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
DEPARTMENT OF CHEMISTRY Requirements For Chemistry Major
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
Quantitative Comparison of the Hydrogen Bond
... hydrogen bond cross-peaks (HNi, C′j, Ni) to the reference peaks (HNi, C′i-1, Ni) (18). To evaluate the reproducibility, a set of six 2D long-range H(N)CO experiments was also recorded at 600 MHz 1H frequency with the same acquisition parameters (measuring time 22 h per experiment). The reported valu ...
... hydrogen bond cross-peaks (HNi, C′j, Ni) to the reference peaks (HNi, C′i-1, Ni) (18). To evaluate the reproducibility, a set of six 2D long-range H(N)CO experiments was also recorded at 600 MHz 1H frequency with the same acquisition parameters (measuring time 22 h per experiment). The reported valu ...
Stoichiometry and the Mole
... involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiometry. Using stoichiometry is a fundamental skill in chemistry; it greatly broadens your ability to pr ...
... involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiometry. Using stoichiometry is a fundamental skill in chemistry; it greatly broadens your ability to pr ...
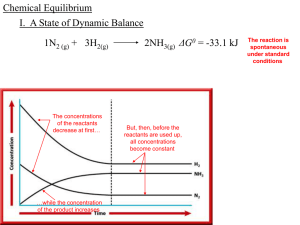
Slide 1
... • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chloride and Keq. ...
... • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chloride and Keq. ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
Chemical Vapor Deposition (CVD)
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
... • This technique is suitable for the manufacture of coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total p ...
Chapter 8 - Chemical Equations and Reactions
... • So many chemical reactions can occur or are occurring that it would be impossible to predict their products if it was not possible to place many of them into categories. • Synthesis reactions are one class of reactions in which substances combine to form a new compound. ...
... • So many chemical reactions can occur or are occurring that it would be impossible to predict their products if it was not possible to place many of them into categories. • Synthesis reactions are one class of reactions in which substances combine to form a new compound. ...
Chapter 8 Notes
... • So many chemical reactions can occur or are occurring that it would be impossible to predict their products if it was not possible to place many of them into categories. • Synthesis reactions are one class of reactions in which substances combine to form a new compound. ...
... • So many chemical reactions can occur or are occurring that it would be impossible to predict their products if it was not possible to place many of them into categories. • Synthesis reactions are one class of reactions in which substances combine to form a new compound. ...
Chemistry: Percent Yield
... Unit 3/4 – Moles / Stoichiometry proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be c ...
... Unit 3/4 – Moles / Stoichiometry proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be c ...
Journal Citation Studies. 46. Physical Chemistry and Chemical
... 10 grouping, with 3,700 citations. In Part 2 of this study we commented that this journal was split into its two parts “only for ease of production .“ According to Young, it really should be considered as one journal. s When we look at Table 3, the top 50 journals most cited by the macrojoumal of ph ...
... 10 grouping, with 3,700 citations. In Part 2 of this study we commented that this journal was split into its two parts “only for ease of production .“ According to Young, it really should be considered as one journal. s When we look at Table 3, the top 50 journals most cited by the macrojoumal of ph ...
7.1 Describing Reactions
... If you examine this equation carefully, you will notice that the number of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical ...
... If you examine this equation carefully, you will notice that the number of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... CHEM.A.2.3.1 – Explain how the periodicity of chemical properties led to the arrangement of elements on the periodic table. Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using thei ...
... CHEM.A.2.3.1 – Explain how the periodicity of chemical properties led to the arrangement of elements on the periodic table. Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using thei ...
Semester 4 - Vaal University of Technology
... The attached logbook makes it easy to keep a permanent record of all your activities during your work integrated learning period. Your tutor/mentor must certify that you satisfactorily performed the work reported. In the event of a change of employer during this period, have your logbook brought up ...
... The attached logbook makes it easy to keep a permanent record of all your activities during your work integrated learning period. Your tutor/mentor must certify that you satisfactorily performed the work reported. In the event of a change of employer during this period, have your logbook brought up ...