Help us improve Wikipedia by supporting it financially
... The lightest elements are hydrogen and helium, both theoretically created by Big Bang nucleosynthesis during the first 20 minutes of the universe[10] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen a ...
... The lightest elements are hydrogen and helium, both theoretically created by Big Bang nucleosynthesis during the first 20 minutes of the universe[10] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen a ...
CHEMISTRY Academic Standards Statement
... substances or to understand how substances are formed and removed in the environment. Chemistry is the science of analysing, transforming or manipulating substances and the molecular interpretation of the world around us. It is at the molecular level that major advances are made in many diverse area ...
... substances or to understand how substances are formed and removed in the environment. Chemistry is the science of analysing, transforming or manipulating substances and the molecular interpretation of the world around us. It is at the molecular level that major advances are made in many diverse area ...
Eperimental studies of V.Ostwald and J.van Hoff
... process (patent 1902), used in the manufacture of nitric acid, although the basic chemistry had been patented some 64 years earlier by Kuhlmann, when it was probably of only academic interest due to the lack of a significant source of ammonia. That may have still been the state of affairs in 1902, a ...
... process (patent 1902), used in the manufacture of nitric acid, although the basic chemistry had been patented some 64 years earlier by Kuhlmann, when it was probably of only academic interest due to the lack of a significant source of ammonia. That may have still been the state of affairs in 1902, a ...
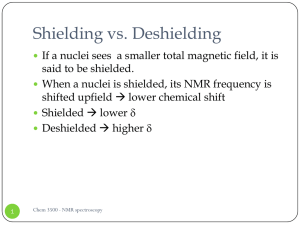
Shielding vs. Deshielding
... Predicting 1H chemical shifts: Shoolery’s rule Several factors can influence the 1H chemical ...
... Predicting 1H chemical shifts: Shoolery’s rule Several factors can influence the 1H chemical ...
Chapter 19 Chemical Thermodynamics
... First Law of Thermodynamics • Energy is neither created nor destroyed. • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. • Energy can, however, be converted from one form to another or transferred fro ...
... First Law of Thermodynamics • Energy is neither created nor destroyed. • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. • Energy can, however, be converted from one form to another or transferred fro ...
LN_ch06
... ►1. Write the correct symbols and formulas for all of the _______ and ____________. ►2. Count the number of each type of _____ on BOTH sides of the __________. ►3. Insert ____________ (numbers to the left of the compound formulas) until there are the equal numbers of each kind of _______ on both sid ...
... ►1. Write the correct symbols and formulas for all of the _______ and ____________. ►2. Count the number of each type of _____ on BOTH sides of the __________. ►3. Insert ____________ (numbers to the left of the compound formulas) until there are the equal numbers of each kind of _______ on both sid ...
Chapter 1: Matter and Change
... ucts that improve our quality of life. applied research. The use of this property to build telecommunications Examples include computers, catalytic networks by sending data on light pulses is the technological development of fiber optics. converters for cars, and biodegradable materials. Technologic ...
... ucts that improve our quality of life. applied research. The use of this property to build telecommunications Examples include computers, catalytic networks by sending data on light pulses is the technological development of fiber optics. converters for cars, and biodegradable materials. Technologic ...
Learning at the symbolic level
... Abstract: The symbolic language of chemistry is extensive, and is used ubiquitously in teaching and learning the subject at secondary level and beyond. This chapter considers how this ‘language’, which acts as such a powerful facilitator of communication for the expert, may often impede effective co ...
... Abstract: The symbolic language of chemistry is extensive, and is used ubiquitously in teaching and learning the subject at secondary level and beyond. This chapter considers how this ‘language’, which acts as such a powerful facilitator of communication for the expert, may often impede effective co ...
Chemistry Unit Outcomes
... List the 4 Principles contained in the Particle Theory of Matter. (Table 1 page 44) Explain what is meant by a pure substance. List 2 examples of pure substances. Explain what is meant by a mixture. List 2 examples of a mixture. Outline from what a solution can be made. List 3 examples of solutions. ...
... List the 4 Principles contained in the Particle Theory of Matter. (Table 1 page 44) Explain what is meant by a pure substance. List 2 examples of pure substances. Explain what is meant by a mixture. List 2 examples of a mixture. Outline from what a solution can be made. List 3 examples of solutions. ...
chemical reaction
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
The Logical Structure of Organic Chemistry and the Empirical
... It is often said that the phase of wave function has no physical significance, because the probabilities for measurement outcomes are the same. Actually, it has chemical significance as is amply exemplified, for instance, by orbital interactions in pericyclic reactions (Woodward & Hoffmann 1969) and ...
... It is often said that the phase of wave function has no physical significance, because the probabilities for measurement outcomes are the same. Actually, it has chemical significance as is amply exemplified, for instance, by orbital interactions in pericyclic reactions (Woodward & Hoffmann 1969) and ...
8 Chemical Equations Chapter Outline Chemical Equations
... 2. Whole number coefficients are placed in front of substances to balance the atoms in the equation. The numbers indicate the units of the substance reacted or formed during the reaction. ...
... 2. Whole number coefficients are placed in front of substances to balance the atoms in the equation. The numbers indicate the units of the substance reacted or formed during the reaction. ...
mc_ch08 - MrBrownsChem1LCHS
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
Chemistry - Plymouth Public Schools
... INQUIRY Scientific literacy can be achieved as students inquire about chemical phenomena. The curriculum should include substantial hands-on laboratory and field experiences, as appropriate, for students to develop and use scientific skills in chemistry, along with the inquiry skills listed below. S ...
... INQUIRY Scientific literacy can be achieved as students inquire about chemical phenomena. The curriculum should include substantial hands-on laboratory and field experiences, as appropriate, for students to develop and use scientific skills in chemistry, along with the inquiry skills listed below. S ...
LECTURE 5 - CHEMICAL EQUILIBRIUM
... equilibrium. Rather, it is said to be metastable. A metastable system is one that changes so slowly that it appears stable. Metastable systems are not at equilibrium but may persist for very long times. The reason that metastable systems exist is the presence of a significant kinetic barrier. Kineti ...
... equilibrium. Rather, it is said to be metastable. A metastable system is one that changes so slowly that it appears stable. Metastable systems are not at equilibrium but may persist for very long times. The reason that metastable systems exist is the presence of a significant kinetic barrier. Kineti ...