VCAA Study Design - Chemistry Education Association
... and Science within the curriculum framework first developed for the Victorian Essential Learning Standards (VELS) - has been designed to ensure that schools and teachers are not required to manage two different curriculum and reporting frameworks during the development of the Australian Curriculum - ...
... and Science within the curriculum framework first developed for the Victorian Essential Learning Standards (VELS) - has been designed to ensure that schools and teachers are not required to manage two different curriculum and reporting frameworks during the development of the Australian Curriculum - ...
20141113080528
... which means that one mole of CO2 has a mass of grams. This relationship yields the following ...
... which means that one mole of CO2 has a mass of grams. This relationship yields the following ...
Introduction_to_Chemical_Reactions_2011
... These compounds break down when put in water. Example: In water, NaCl Na1+ and Cl1-. We say that NaCl… has dissolved. is soluble. forms an aqueous solution (aq). ...
... These compounds break down when put in water. Example: In water, NaCl Na1+ and Cl1-. We say that NaCl… has dissolved. is soluble. forms an aqueous solution (aq). ...
How do we predict chemical change?
... Not every combination of substances will lead to the formation of new compounds via a chemical reaction. How can we predict when a chemical process takes place? One approach could be to compare the relative stability of reactants and products. We might expect that chemical reactions will proceed in ...
... Not every combination of substances will lead to the formation of new compounds via a chemical reaction. How can we predict when a chemical process takes place? One approach could be to compare the relative stability of reactants and products. We might expect that chemical reactions will proceed in ...
Chemical Reaction
... • Catalyst: a substance that increases the rate of chemical reaction. • Inhibitor: a substance that decreases the rate of chemical reaction. • Law of Conservation of mass: states that during a chemical reaction or a physical change, mass is not created or destroyed but transformed into a new substan ...
... • Catalyst: a substance that increases the rate of chemical reaction. • Inhibitor: a substance that decreases the rate of chemical reaction. • Law of Conservation of mass: states that during a chemical reaction or a physical change, mass is not created or destroyed but transformed into a new substan ...
preliminary course outline facilitators course description
... Obtain explicit consent before any type of audio-visual recording can take place. No unauthorized distribution of any audio-visual recording is allowed. No unauthorized distribution of any course material is allowed. This includes copies of examinations, assignments and electronic data associated wi ...
... Obtain explicit consent before any type of audio-visual recording can take place. No unauthorized distribution of any audio-visual recording is allowed. No unauthorized distribution of any course material is allowed. This includes copies of examinations, assignments and electronic data associated wi ...
The Chemist - American Institute of Chemists
... caused by ‘drugs of dependence’, such as heroin, which destroy human lives; however, they are at the same time unaware that morphine, which is widely used to relieve pain, is closely related chemically to heroin. It is this lack of association of ‘drugs’ with beneficial chemical activity which in pa ...
... caused by ‘drugs of dependence’, such as heroin, which destroy human lives; however, they are at the same time unaware that morphine, which is widely used to relieve pain, is closely related chemically to heroin. It is this lack of association of ‘drugs’ with beneficial chemical activity which in pa ...
The masses of reactants and products are equal.
... conservation of mass using combination notes. ...
... conservation of mass using combination notes. ...
Additional Review
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
chemical reaction
... Types of Reactions • Single _____________ reaction in which one element takes the place of another element in a compound. • (One person breaks up a couple and goes out with one of them. • A + BC→ AC + B ...
... Types of Reactions • Single _____________ reaction in which one element takes the place of another element in a compound. • (One person breaks up a couple and goes out with one of them. • A + BC→ AC + B ...
LESSON 23: Exploding Bags
... properties are color, shape, boiling point, melting point, and density. Chemical properties can be identified by observing how a chemical reacts with other substances. Some examples of chemical properties include acidity, toxicity, and flammability. During the experiment, students can observe the di ...
... properties are color, shape, boiling point, melting point, and density. Chemical properties can be identified by observing how a chemical reacts with other substances. Some examples of chemical properties include acidity, toxicity, and flammability. During the experiment, students can observe the di ...
PHYSICAL PROPERTIES - can observe w/o changing the
... atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to watch for) formation of an odor, change in temp, formation ...
... atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to watch for) formation of an odor, change in temp, formation ...
Teacher Background - Online Learning Exchange
... the mole ratio and then skip the mass-mole conversion step. Stress that because the number of grams in one mole of a substance varies with its molar mass, a mass-mole conversion is a necessary intermediate step in mass-mass stoichiometric problems. Caution students not to make quick judgments when f ...
... the mole ratio and then skip the mass-mole conversion step. Stress that because the number of grams in one mole of a substance varies with its molar mass, a mass-mole conversion is a necessary intermediate step in mass-mass stoichiometric problems. Caution students not to make quick judgments when f ...
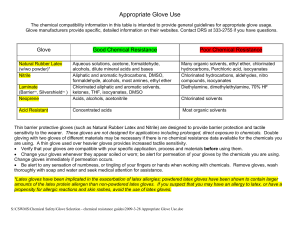
Appropriate Glove Use
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, ...
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, ...