* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Matter - cloudfront.net
Gas chromatography wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical element wikipedia , lookup
Water pollution wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Stoichiometry wikipedia , lookup
Drug discovery wikipedia , lookup
Safety data sheet wikipedia , lookup
Condensed matter physics wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
History of chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Atomic theory wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
VX (nerve agent) wikipedia , lookup
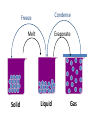
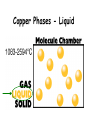
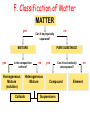
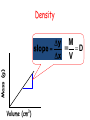
IQ 1 1. Define “Matter” 2. What 2 major groups is matter divided into? 3. Give an example of a heterogeneous and homogeneous mixture. Unit 2: Matter and Density Chapter 2, 3-4 I. Matter NOTE: Matter= anything that: a) has mass, and b) takes up space NOTE: Mass = a measure of the amount of “stuff” (or material) the object contains (don’t confuse this with weight, a measure of gravity) NOTE: Volume = a measure of the space occupied by the object A. Describing Matter 1. Properties used to describe matter can be classified as: a. Extensive – depends on the amount of matter in the sample - Mass, volume, calories are examples b. Intensive – depends on the type of matter, not the amount present - Hardness, Density, Boiling Point B. Properties are… 1. Words that describe matter (adjectives) 2. Physical Properties- a property that can be observed and measured without 3. changing the material’s composition. - Examples- color, hardness, m.p., b.p. 4. Chemical Properties- a property that can only be observed by changing the composition of the material. - Examples- ability to burn, decompose, ferment, react with, etc. C. States of matter 1. Solid- matter that can not flow (definite shape) and has definite volume. 2. Liquid- definite volume but takes the shape of its container (flows). 3. Gas- a substance without definite volume or shape and can flow. a. Vapor- a substance that is currently a gas, but normally is a liquid or solid at room temperature. (Which is correct: “water gas”, or “water vapor”?) 4. 4th state: Plasma - formed at high temperatures; ionized phase of matter as found in the suns States of Matter Definite Volume? Solid Liquid Gas YES YES NO Result of a DefiniteTemperature Shape? Increase? Will it Compress? YES Small Expans. NO NO Small Expans. NO NO Large Expans. YES Three Main Phases – page 41 Condense Freeze Evaporate Melt Solid Liquid Gas Copper Phases - Solid Copper Phases - Liquid Copper Phases – Vapor (gas) D. Physical vs. Chemical Change 1. Physical change will change the visible appearance, without changing the composition of the material. a. Boil, melt, cut, bend, split, crack b. Is boiled water still water? 2. Can be reversible, or irreversible. 3. Chemical change - a change where a new form of matter is formed. – Rust, burn, decompose, ferment E. Mixtures 1. Mixtures are a physical blend of at least two substances; have variable composition. They can be either: a. Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. b. Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water 2. Every part keeps it’s own properties. 3. Solutions are homogeneous mixtures 4. Mixed molecule by molecule, thus too small to see the different parts 5. Can occur between any state of matter: gas in gas; liquid in gas; gas in liquid; solid in liquid; solid in solid (alloys), etc. 6. Thus, based on the distribution of their components, mixtures are called homogeneous or heterogeneous. F. Phase 1. The term “phase” is used to describe any part of a sample with uniform composition of properties. 2. A homogeneous mixture consists of a single phase 3. A heterogeneous mixture consists of two or more phases. 4. Note Figure 2.6, page 45 2.2 Olive oil and vinegar are homogeneous mixtures. When olive oil is mixed with vinegar, they form a heterogeneous mixture with two distinct phases. Fig. 2.6 pg. 45 G. Separating Mixtures 1. Some can be separated easily by physical means: rocks and marbles, iron filings and sulfur (use magnet) 2. Differences in physical properties can be used to separate mixtures. 3. Filtration - separates a solid from the liquid in a heterogeneous mixture (by size) – (Fig. 2.7, page 46) 2.2 Separating Mixtures A colander is used to separate pasta from the water in which it was cooked. This process is a type of filtration. Figure 2.7, page 46 2.2 Separating Mixtures During a distillation, a liquid is boiled to produce a vapor that is then condensed into a liquid. Figure 2.8, page 47 Distillation: takes advantage of different boiling points. NaCl boils at 1415 oC Separation by Physical Means magnet Separation of a Mixture Components of dyes such as ink may be separated by paper chromatography. II. Substances NOTE: Substances are either: a) elements, or b) compounds A. Substances: element or compound 1. Elements- simplest kind of matter a. cannot be broken down any simpler and still have properties of that element! b. all one kind of atom. 2. Compounds are substances that can be broken down only by chemical methods a. when broken down, the pieces have completely different properties than the original compound. b. made of two or more atoms, chemically combined (not just a physical blend!) B. Compound vs. Mixture Compound Mixture Made of one kind of material Made of more than one kind of material Made by a chemical change Made by a physical change Definite composition Variable composition Which is it? Mixture Element Compound C. Elements vs. Compounds 1. Compounds can be broken down into simpler substances by chemical means, but elements cannot. 2. A “chemical change” is a change that produces matter with a different composition than the original matter. D. Chemical Change 1. A change in which one or more substances are converted into different substances. 2. Heat and light are often evidence of a chemical change. E. Properties of Compounds 1. Quite different properties than their component elements. 2. Due to a CHEMICAL CHANGE, the resulting compound has new and different properties: a. Table sugar – carbon, hydrogen, oxygen b. Sodium chloride – sodium, chlorine c. Water – hydrogen, oxygen F. Classification of Matter Matter Flowchart MATTER yes no Can it be physically separated? MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids yes Can it be chemically decomposed? Compound Suspensions no Element G. Symbols & Formulas 1. Currently, there are 118 elements 2. Elements have a 1 or two letter symbol, and compounds have a formula. 3. An element’s first letter always capitalized; if there is a second letter, it is written lowercase: B, Ba, C, Ca, H, He 4. Start learning the elements names and symbols listed in Table B.7 on page R53 5. Some names come from Latin or other languages; note Table 2.2, page 52 Elements named after Latin (old). a. Sodium b. Gold c. Silver d. Potassium e. Lead f. Antimony g. Iron h. Tungsten i. Tin j. Copper k. Mercury _____ Na _____ Au Ag _____ _____ K _____ Pb _____ Sb _____ Fe _____ W _____ Sn _____ Cu _____ Hg _______________________ Natrium _______________________ Aurum Argentium _______________________ _______________________ Kalium _______________________ Plumbum _______________________ Stibinite _______________________ Ferrum _______________________ Wolfram _______________________ Stannum _______________________ Cuprum Hydrogyrum _______________________ Element Quiz #1 1) Ag 2) Nitrogen 3) Oxygen 4) Mg 5) Helium 6) F 7) Zn 8) H 9) Carbon 10)Mercury Silver N O Magnesium He Fluorine Zinc Hydrogen C Hg 1) Ag 2) Nitrogen 3) Oxygen 4) Mg 5) Helium 6) F 7) Zn 8) H 9) Carbon 10)Mercury Element quiz 1 Element Quiz #2 1) Zn 2) Strontium 3) Manganese 4) Se 5) Argon 6) Fe 7) Au 8) Be 9) Silicon 10)Lead Zinc Sr Mn Selenium Ar Iron Gold Beryllium Si Pb Element Quiz #2 1) Sr 2) Boron 3) Bromine 4) P 5) Iodine 6) F 7) Cu 8) Ba 9) U 10)Calcium Strontium B Br Phosphorus I Fluorine Copper Barium Uranium Ca H. Extensive vs. Intensive • Examples: 1. boiling point intensive 2. volume extensive 3. mass extensive 4. density intensive 5. conductivity intensive I . Physical vs. Chemical Properties • Examples: 1. melting point physical 2. flammable chemical 3. density physical 4. magnetic physical 5. tarnishes in air chemical J. Physical vs. Chemical Change • Examples: 1. rusting iron chemical 2. dissolving in water physical 3. burning a log chemical 4. melting ice physical 5. grinding spices physical Basic Vinaigrette Recipe • • • • • • • 1 shallot finely minced 1 tsp. dijon mustard (weak emulsifiers) ¾ tsp. mayonnaise (contains lectithin- emulsifier) 7 tsp. vinegar (basalmic or other acid) ¼ cup olive oil ½ tsp. honey (optional) Salt and pepper – Fresh herbs can be added if desired Mix shallot, honey, vinegar, dijon, and mayo until smooth. Slowly drizzle in ¼ cup of light or regular olive oil (other neutral flavored oils will work fine, but avoid extra virgin olive oil as the flavor can be overpowering. Season with salt and pepper to taste. K. Chemical Changes 1. The ability of a substance to undergo a specific chemical change is called a chemical property. a. iron plus oxygen forms rust, so the ability to rust is a chemical property of iron. 2. During a chemical change (also called chemical reaction), the composition of matter always changes. L. Chemical Reactions are… 1. When one or more substances are changed into new substances. 2. Reactants- the stuff you start with 3. Products- what you make 4. The products will have NEW PROPERTIES different from the reactants you started with 5. Arrow points from the reactants to the new products M. Recognizing Chemical Changes 1. Energy is absorbed or released (temperature changes hotter or colder) 2. Color changes 3. Gas production (bubbling, fizzing, or odor change; smoke) 4. formation of a precipitate - a solid that separates from solution (won’t dissolve) 5. Irreversibility - not easily reversed NOTE: But, there are examples of these that are not chemical – boiling water bubbles, etc. N. Conservation of Mass 1. During any chemical reaction, the mass of the products is always equal to the mass of the reactants. 2. All of the mass can be accounted for: a. Burning of wood results in products that appear to have less mass as ashes; where is the rest? 3. Law of Conservation of Mass - Page 55 43.43 g Original mass reactants = 43.43 g Final mass = product II. DENSITY A. Density is an intensive property of matter. Styrofoam 1. does NOT depend on quantity of matter. 2. temperature B. Contrast with extensive 1. depends on quantity of matter. 2. mass and volume. Brick Density Mass (g) M = D V Volume (cm3) C. Density Problems: 1. An object has a volume of 825 cm3 and a density of 13.6 g/cm3. Find its mass. GIVEN: V = 825 cm3 D = 13.6 g/cm3 M=? M D= V WORK: M = DV M =(13.6 g/cm3)(825cm3) M = 11,200 g 2. A liquid has a density of 0.87 g/mL. What volume is occupied by 25 g of the liquid? GIVEN: D = 0.87 g/mL V=? M = 25 g M D V WORK: V=M D V= 25 g 0.87 g/mL V = 29 mL 3. The density of gold is 19.3 g/mL. Calculate the density of gold in (a) lb/ft3, (b) kg/m3. g (a) mL lb cm3 in ft 3 3 12 in 19.3 g 1 lb 1 mL 2.54 cm x x x x 3 in 1 ft 453.6 g 1 cm 1 mL kg (b) g cm3 m3 19.3 g 1 kg x 3 103 g cm = 1204.8 lb/ft3 x 1 cm 10-2 m = 19,300 kg/m3 = 1.20 x 103 lb/ft3 3 = 1.93 x 104 kg/m3 D. More Density Calculations 1. An object has a mass of 1.00 kg and a density of 4.00 g/mL. What is its volume in liters? m m = 1.00 kg V m = 1000 g D D = 4.00 g/mL 1000 g = 250 mL V m D 4.00 g/mL V = 0.250 L 2. What is the mass of a 500 cm3 object that has a specific gravity of 0.800? V = 500 cm3 sp.gr. = 0.800 m DV D = 0.800 m D V g/cm3 m (0.800 g/cm )(500 cm ) 3 = 400 g 3 *It is often required to substitute one or more equations into another equation to form the working equation. **If the radius, mass, and density of a cylinder are know, the equation for the height of the cylinder can be formed by combining and rearranging the density and volume formulae as follows: Step 1: m 2 D ; Vπr h V Step 2: m D 2 πr h m h 2 πr D If the length, width, height, and density of a block of metal are known, show the equation that can be used to calculate its mass. m D V m=D•V m=D•l•w•h V=l•w•h If the radius and mass of a metal ball are known, show the equation that can be used to determine its density. m D V 4 V πr 3 D 3 m 4 3 πr 3 3m D 4π r 3 The dimensions of a swimming pool are 13.5 ft. x 22 m x 225 cm. (a) Determine the volume of the pool in m3. (b) Determine the mass of the water in pounds. (a) V = l · w · d 13.5 ft x 12 in 1 ft x 1 m 39.37 in V = 4.1148 m · 22 m · 2.25 m = 4.1148 m = 203.68 m3 = 2.0 x 102 m3 (b) 203.68 m3 3 x 1 cm 10-2 m x 1 g 1 cm3 x 1 lb 453.6 g = 4.5 x 105 lb Learning Check Osmium is a very dense metal. What is its density in g/cm3 if 50.00 g of the metal occupies a volume of 2.22cm3? 1) 2.25 g/cm3 2) 22.5 g/cm3 3) 111 g/cm3 Solution Placing the mass and volume of the osmium metal into the density setup, we obtain D = mass = 50.00 g = volume 2.22 cm3 = 22.522522 g/cm3 = 22.5 g/cm3 Volume Displacement A solid displaces a matching volume of water when the solid is placed in water. 33 mL 25 mL Learning Check What is the density (g/cm3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 mL to 33 mL? 1) 0.2 g/ cm3 2) 6 g/cm3 3) 252 g/cm3 33 mL 25 mL Learning Check Which diagram represents the liquid layers in the cylinder? (K) Karo syrup (1.4 g/mL), (V) vegetable oil (0.91 g/mL,) (W) water (1.0 g/mL) 1) 2) 3) V K W W K V K V W Learning Check The density of octane, a component of gasoline, is 0.702 g/mL. What is the mass, in kg, of 875 mL of octane? 1) 0.614 kg 2) 614 kg 3) 1.25 kg Learning Check If blood has a density of 1.05 g/mL, how many liters of blood are donated if 575 g of blood are given? 1) 0.548 L 2) 1.25 L 3) 1.83 L Learning Check A group of students collected 125 empty aluminum cans to take to the recycling center. If 21 cans make 1.0 pound of aluminum, how many liters of aluminum (D=2.70 g/cm3) are obtained from the cans? 1) 1.0 L 2) 2.0 L 3) 4.0 L Density Lab (No Uncertainty) Station A: Water 1. Mass of empty cylinder 2. Mass of cylinder & water Throw Paper Towels Away/ Pour water back in beaker/ Don’t turn on the sinks Station B: Unknown liquid (Repeat Station A)(Pour back in bottle & PUT LID ON) Station C: Dimensions & mass. Station D: Copper (DO NOT LOSE!!!!!)(Dimensions & mass) Station E. Irregular Shape (completely submerge object) Object one: Rubber Stopper Object two: Nut & bolt (Careful) Accepted density = 7.87 Station F: Determine density of Al cyl. by H2O displacement. Determine thickness of foil.