Occurrence and Origin of Andalusite in Peraluminous Felsic Igneous
... more textural or chemical criteria suggesting a magmatic origin. Of the many possible controls on the formation of andalusite (excess Al2O3, water concentration and fluid evolution, high Be–B–Li–P, high F, high Fe–Mn–Ti, and kinetic considerations), the two most important factors appear to be excess ...
... more textural or chemical criteria suggesting a magmatic origin. Of the many possible controls on the formation of andalusite (excess Al2O3, water concentration and fluid evolution, high Be–B–Li–P, high F, high Fe–Mn–Ti, and kinetic considerations), the two most important factors appear to be excess ...
Occurrence and Origin of Andalusite in
... crystallization from a silicate melt, and (d) pegmatitic (watersaturated, T#), associated with aplite–pegmatite contacts or pegmatitic portion alone; Type 3 Metasomatic—(water-saturated, magma-absent), spatially related to structural discontinuities in host, replacement of feldspar and/or biotite, i ...
... crystallization from a silicate melt, and (d) pegmatitic (watersaturated, T#), associated with aplite–pegmatite contacts or pegmatitic portion alone; Type 3 Metasomatic—(water-saturated, magma-absent), spatially related to structural discontinuities in host, replacement of feldspar and/or biotite, i ...
Unit 10A Stoichiometry Notes
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2H4 + N2O4 → 3 N2 + 4 H2O b. How many moles of N2 will be produced if 2 ...
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2H4 + N2O4 → 3 N2 + 4 H2O b. How many moles of N2 will be produced if 2 ...
Unit 9 Stoichiometry Notes
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2H4 + N2O4 → 3 N2 + 4 H2O ...
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2H4 + N2O4 → 3 N2 + 4 H2O ...
Unit 8 Stoichiometry Notes
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2 H 4 + N 2 O 4 → 3 N 2 + 4 H 2 O b. How many moles of N2 will be produ ...
... 5. A reaction between hydrazine, N2H4 , and dinitrogen tetroxide, N2O4 , has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor. a. Write a balanced chemical equation for this reaction. 2 N2 H 4 + N 2 O 4 → 3 N 2 + 4 H 2 O b. How many moles of N2 will be produ ...
CO2 Capture from Flue gas using Amino acid salt
... storage (CCS) [4,5]. It is generally estimated that the capture part represents about 80% of the global cost of CCS, while the rest is distributed between transport and storage [6]. CO2 capture is the focus of this project. CO2 capture has the potential for large reductions (approximately 80-90%) of ...
... storage (CCS) [4,5]. It is generally estimated that the capture part represents about 80% of the global cost of CCS, while the rest is distributed between transport and storage [6]. CO2 capture is the focus of this project. CO2 capture has the potential for large reductions (approximately 80-90%) of ...
Chapter 10 Chemical Calculations and Chemical Equations
... 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more important than others are, and the amounts ...
... 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more important than others are, and the amounts ...
Study Guide Chapter 10: An Introduction to Chemistry
... 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more important than others are, and the amounts ...
... 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more important than others are, and the amounts ...
Chemistry Final Exam Review
... a. available amount of one of the reactants. c. available amount of each reactant. b. amount of product formed. d. speed of the reaction. ____ 130. According to the kinetic-molecular theory, particles of matter are in motion in a. gases only. c. solids, liquids, and gases. b. gases and liquids. d. s ...
... a. available amount of one of the reactants. c. available amount of each reactant. b. amount of product formed. d. speed of the reaction. ____ 130. According to the kinetic-molecular theory, particles of matter are in motion in a. gases only. c. solids, liquids, and gases. b. gases and liquids. d. s ...
CHEMICAL AND PROCESS DESIGN HANDBOOK
... Chemicals are part of our everyday lives. The hundreds of chemicals that are manufactured by industrial processes influence what we do and how we do it. This book offers descriptions and process details of the most popular of those chemicals. The manufacture of chemicals involves many facets of chem ...
... Chemicals are part of our everyday lives. The hundreds of chemicals that are manufactured by industrial processes influence what we do and how we do it. This book offers descriptions and process details of the most popular of those chemicals. The manufacture of chemicals involves many facets of chem ...
"Cyano Compounds, Inorganic," in: Ullmann`s Encyclopedia of
... (2) well-tested technology, with a simple and safe reaction system; and (3) high-purity HCN. A disadvantage is that the process is dependent on pure methane as a raw material to avoid carburization of the platinum catalyst; for example, a small percentage of higher hydrocarbon impurities rapidly cau ...
... (2) well-tested technology, with a simple and safe reaction system; and (3) high-purity HCN. A disadvantage is that the process is dependent on pure methane as a raw material to avoid carburization of the platinum catalyst; for example, a small percentage of higher hydrocarbon impurities rapidly cau ...
1 – Introduction
... The percentage yield of K2O from the original 100g. of fertilizer is the number shown on the label. A potash fertilizer would thus be labeled 0-0-60, not 0-0-52. 4 - History of fertilizer While manure, cinder and iron making slag have been used to improve crops for centuries, use of inorganic artifi ...
... The percentage yield of K2O from the original 100g. of fertilizer is the number shown on the label. A potash fertilizer would thus be labeled 0-0-60, not 0-0-52. 4 - History of fertilizer While manure, cinder and iron making slag have been used to improve crops for centuries, use of inorganic artifi ...
33 POLYMERS I OPTIONAL MODULE - 2
... study of another vast area i.e. polymers. Today polymers have influenced our life style to the extent that it would not be wrong to say that we are in polymer age. Now-a-days polymers find wide range of uses starting from common household utensils, automobiles, clothes, furniture, etc., to space-air ...
... study of another vast area i.e. polymers. Today polymers have influenced our life style to the extent that it would not be wrong to say that we are in polymer age. Now-a-days polymers find wide range of uses starting from common household utensils, automobiles, clothes, furniture, etc., to space-air ...
Removal of hydrogen fluoride from gas streams
... were assessed for HF removal in the presence of fluorine. Other inorganic adsorbent were also investigated e.g. mixtures of NaF with other metal fluorides and an aluminophosphate molecular sieve. It was noted that most of the calcium salts do have a capacity for HF, but can release their anions as i ...
... were assessed for HF removal in the presence of fluorine. Other inorganic adsorbent were also investigated e.g. mixtures of NaF with other metal fluorides and an aluminophosphate molecular sieve. It was noted that most of the calcium salts do have a capacity for HF, but can release their anions as i ...
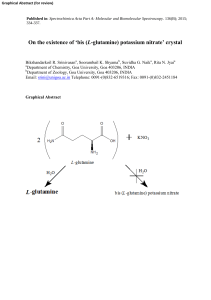
`bis (L-glutamine) potassium nitrate` crystal
... chemical formula. Many so called novel NLO materials are often represented by an unusual name for example bis (L-glutamine) potassium nitrate [11], abbreviated by a strange code. L-glutamine is one of the twenty naturally occurring amino acids and is an amide of Lglutamic acid. The compound monosodi ...
... chemical formula. Many so called novel NLO materials are often represented by an unusual name for example bis (L-glutamine) potassium nitrate [11], abbreviated by a strange code. L-glutamine is one of the twenty naturally occurring amino acids and is an amide of Lglutamic acid. The compound monosodi ...
Catalysts Containing Depleted Uranium Compounds
... poisons (for example, sulphur, water, chlorine, coke) and the opportunity of replacing expensive industrial catalysts based on precious metals by more efficient catalysts such as UOx. PHYSICOCHEMICAL FEATURES OF URANIUM OXIDES ...
... poisons (for example, sulphur, water, chlorine, coke) and the opportunity of replacing expensive industrial catalysts based on precious metals by more efficient catalysts such as UOx. PHYSICOCHEMICAL FEATURES OF URANIUM OXIDES ...
LaBrake, Fundamentals Diagnostic Questions
... 16. Iced tea with lemon, ice, and sugar is an example of a) a heterogeneous mixture (correct) b) a homogenous mixture c) a mineral d) an element e) a compound 17. All of the following are considered subatomic particles, except: a) gamma rays (correct) b) electrons c) protons d) neutrons e) positron ...
... 16. Iced tea with lemon, ice, and sugar is an example of a) a heterogeneous mixture (correct) b) a homogenous mixture c) a mineral d) an element e) a compound 17. All of the following are considered subatomic particles, except: a) gamma rays (correct) b) electrons c) protons d) neutrons e) positron ...
Rubidium
... the element in commercially significant amounts. One notable source is also in the extensive deposits of pollucite at Bernic Lake, Manitoba. Rubidium metal can be produced by reducing rubidium chloride with calcium among other methods. ...
... the element in commercially significant amounts. One notable source is also in the extensive deposits of pollucite at Bernic Lake, Manitoba. Rubidium metal can be produced by reducing rubidium chloride with calcium among other methods. ...
A Dictionary of the New Chymical Nomenclature
... Magnesian earth Magnesia alba, of the shops Bergman's aerated magnesia Cretaceous magnesia ...
... Magnesian earth Magnesia alba, of the shops Bergman's aerated magnesia Cretaceous magnesia ...
www.iitvidya.com salt analysis assignment 1. A compound on
... 3 (B) Elementals (C) 37. Identify the following : Na2CO3 SO (D) Also mention the oxidation state of S in all the compounds : 38. How is boron obtained from borax ? Give chemical equations with reaction conditions ? Write the structure of B2H6 and its reaction with HCl. 39. A metallic c ...
... 3 (B) Elementals (C) 37. Identify the following : Na2CO3 SO (D) Also mention the oxidation state of S in all the compounds : 38. How is boron obtained from borax ? Give chemical equations with reaction conditions ? Write the structure of B2H6 and its reaction with HCl. 39. A metallic c ...
kcse chemistry questions
... State what would be observed when dilute hydrochloric acid is added to the products formed when a mixture of iron fillings and sulphur? (1mk) Describe how the following reagents can be used to prepare lead sulphate solid potassium sulphate, solid lead carbonate, dilute nitric acid and distilled wate ...
... State what would be observed when dilute hydrochloric acid is added to the products formed when a mixture of iron fillings and sulphur? (1mk) Describe how the following reagents can be used to prepare lead sulphate solid potassium sulphate, solid lead carbonate, dilute nitric acid and distilled wate ...
Chapter 22 - 2012 Book Archive
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
Chapter 09 An Overview of Chemical Reactions Notes
... b. How many moles of carbon dioxide would be produced if 2.5 mol of calcium carbonate is used? 4. Aluminum reacts with hydrochloric acid, HCl (aq), to produce aluminum chloride and hydrogen gas. a. Write a balanced equation for the reaction. b. Calculate the number of moles of HCl (aq) required to r ...
... b. How many moles of carbon dioxide would be produced if 2.5 mol of calcium carbonate is used? 4. Aluminum reacts with hydrochloric acid, HCl (aq), to produce aluminum chloride and hydrogen gas. a. Write a balanced equation for the reaction. b. Calculate the number of moles of HCl (aq) required to r ...
Study of C4F8 `N2 and C4F8 `Ar`N2 plasmas for highly
... We report the effect of N2 addition to C4 F8 and C4 F8 /Ar discharges on plasma etching rates of organosilicate glass 共OSG兲 and etch stop layer materials (Si3 N4 and SiC兲, and the results of surface chemistry studies performed in parallel. N2 addition exhibits different effects in C4 F8 and C4 F8 /A ...
... We report the effect of N2 addition to C4 F8 and C4 F8 /Ar discharges on plasma etching rates of organosilicate glass 共OSG兲 and etch stop layer materials (Si3 N4 and SiC兲, and the results of surface chemistry studies performed in parallel. N2 addition exhibits different effects in C4 F8 and C4 F8 /A ...
Fluorochemical industry
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining (the main source of fluorine) was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.Mined fluorite is separated into two main grades, with about equal production of each. Acidspar is at least 97% CaF2; metspar is much lower purity, 60–85%. (A small amount of the intermediate, ceramic, grade is also made.) Metspar is used almost exclusively for iron smelting. Acidspar is primarily converted to hydrofluoric acid (by reaction with sulfuric acid). The resultant HF is mostly used to produce organofluorides and synthetic cryolite.