* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHEMICAL REACTIONS Chapter 4
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Computational chemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Isotopic labeling wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Water splitting wikipedia , lookup
Biochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Click chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Electrochemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical potential wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Rate equation wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical Corps wikipedia , lookup
Safety data sheet wikipedia , lookup
Transition state theory wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
VX (nerve agent) wikipedia , lookup
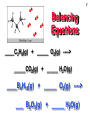
1 CHEMICAL REACTIONS Chapter 4 Reactants: Zn + I2 Product: Zn I2 Chemical Equations 2 4 Al(s) + 3 O2(g) ---> 2 Al2O3(s) This equation means 4 Al atoms + 3 O2 molecules ------> 2 formula units of Al2O3 4 moles of Al + 3 moles of O2 ----> 2 moles of Al2O3 Chemical Equations Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al(s) + 3 O2(g) ---> 2 Al2O3(s) The numbers in the front are called stoichiometric coefficients The letters (s), (g), and (l) are the physical states of compounds. 3 Chemical Equations Because of the principle of the Lavoisier, 1788 conservation of matter, an equation must be balanced. It must have the same number of atoms of the same kind on both sides. 4 Chemical Equations •The Law of the Conservation of Matter • The amount of matter in a system does not change: the same atoms are present in a reaction at the beginning and at the end. 5 6 Balancing Equations ___ Al(s) + ___ Br2(liq) ---> ___ Al2Br6(s) 7 Balancing Equations ____C3H8(g) + _____ O2(g) ----> _____CO2(g) + _____ H2O(g) ____B4H10(g) + _____ O2(g) ----> ___ B2O3(g) + _____ H2O(g) 8 STOICHIOMETRY - the study of the quantitative aspects of chemical reactions. STOICHIOMETRY It rests on the principle of the conservation of matter. 2 Al(s) + 3 Br2(liq) ------> Al2Br6(s) 9 10 STOICHIOMETRY CALCULATIONS Stoichiometric factor Moles reactant Moles product Zn + 2 HCl ZnCl2+ H2 Given 7 mol of Zn, 1. How many moles of acid are need to react all the zinc? 2. How many moles of hydrogen gas are produced? 3.How many moles of ZnCl2 are produced? 2 KClO3 2 KCl + 3 O2 Given you need to produce 21 moles of oxygen gas: 1. How many moles of KClO3 do you need to start with? 2. How many moles of KCl are produced? 2 2H6+ 7 O2 4 CO2+ 6 H2O __C Given 10 moles of C2H6 : 1. How many mole of oxygen gas do you need to react all the ethane? 2. How many moles of water are produced? 3. How many moles of carbon dioxide will be produced?