* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Minerals on the Go
Siderophore wikipedia , lookup
NMDA receptor wikipedia , lookup
Protein adsorption wikipedia , lookup
Index of biochemistry articles wikipedia , lookup
Membrane potential wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Western blot wikipedia , lookup
Lipid signaling wikipedia , lookup
Proteolysis wikipedia , lookup
Magnesium in biology wikipedia , lookup
Cell membrane wikipedia , lookup
Magnesium transporter wikipedia , lookup
Paracrine signalling wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Endomembrane system wikipedia , lookup
Molecular neuroscience wikipedia , lookup
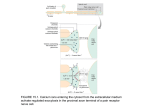
Minerals on the Move Transport in the Blood and Cellular Uptake Postabsorption (postprandial) events in mineral nutrition 1. Transport in the blood 2. Movement across membranes 3. Intracellular location Rules to Consider Rule: Whereas macrominerals (Ca2+, Mg2+, Na+, Cl- etc.) travel in the blood and access cells primarily as free ions, the micronutrients (Cu2+, Zn2+, Fe2+, Mn2,, Se) rely on proteins and other ligands for transport and delivery Rule: Targeting microminerals to select organs and locations within cells is a function of transport proteins in concert with low Mwt ligands. Rule: Common to both macro-and microminerals are specific portals or gateways to channels in the membranes through which minerals can move from outside to inside the cell. The activity of these portals is carefully regulated. Rule: In general, because of their bulk, macrominerals use the energy of diffusion to gain access to the cytosol from the extracellular fluid, microminerals require energy-driven transport mechanisms. Major Blood Minerals Total (mmol/L) Calcium 2.5 Bound (mmol/L) % Bound 1.0 – 1.5 46 Magnesium 0.48-0.66 0.1 – 0.3 32 Potassium 3.5 – 5.0 minor _ 135 - 145 minor _ Sodium Chloride Phosphate 98 - 108 0.7 – 1.4 minor 0.5 – 1.0 _ 70 (phospholipids) Transport in the Blood The objective of blood transport is to relocate an absorbed mineral to its action site or storage site Transport proteins generally work with receptors on cell membranes A variety of transport carriers have been identified for both macroand microminerals Fe (non heme) Transferrin Zn -2 macroglobulin, albumin Cu Ceruloplasmin, serum albumin Mn Transferrin Ca Albumin (50% protein bound) Mg Albumin (32% protein bound) Se Selenoprotein P Table 1. Distribution and Kinetics of Body Iron Compartment Iron (grams) Percent of Total Hemoglobin 2.7 66 Myoglobin 0.2 3 0.008 0.1 Non-heme Enzymes < 0.0001 --- Intracellular Storage (Ferritin) 1.0 30 Intracellular Labile Iron (Chelatable Iron) 0.07 (?) 1 Intercellular Transport (Transferrin) 0.003 0.1 Heme Enzymes Total 3.98 grams Typical transport protein Membrane receptor recognition site Metal-binding sites Revealing a secret molecular handshake. A new model shows that iron-transporting transferrins bind to the side and underside of the transferrin receptor, not to the top. The transferrin molecule straightens when it attaches to the receptor, which puts the C-lobe in a position to more quickly release its iron load, while the N-lobe may have a harder time letting go of its metal cargo. Transferrin iron enters via endocytosis Receptor recognitionbinding site Transmembrane Transport Membrane receptors Gated channels ATP-driven transport Endocytosis 4 ways Minerals penetrate the Cell Membrane • Simple Diffusion • Passive transport (facilitated diffusion) • Active transport (energy-dependent) • Receptor-mediated endocytosis Simple Diffusion Initial Final High Low Ficks Law of diffusion: The rate of diffusion of an ion at steadystate transmembrane flux varies inversely with path length and directly with area and concentration gradient Unbound Ca = 1.0 mM ADca in blood ([Ca]1 – [Ca]2) F= L Cytosolic Ca = 0.00001 mM 2 A = 80 m L = 10 m Dca = 3 x 10-3 cm2/min after Bronner Adolph Fick Based on Fick’s law, the expected diffusion rate of Ca across the intestinal cell is 96 x 10-18 mol/min/cell. Rate observed is 70 times greater at Vmax, which means duodenal cells have factors that enhance self diffusion of Ca Factor identified as Calbindin, a small (9 kD) Ca-binding protein Facilitated Diffusion (Mediated Transport) Calcium Channels Topology of a calcium channel sitting in the cell membrane. Just a mutation in one of the 2,000-plus amino acids (red dot) disrupts the molecule's shut-off mechanism and allows abnormal calcium entry into cells. A molecular model of a calcium channel protein (purple helices) complexed with the drug nifedipine in the middle of the pore. The orange spheres are isoleucine residues that act as a "gate" for the passage of calcium ions (yellow spheres). Drugs may close the gate and prevent the influx of calcium. Such channel-blocking drugs help reduce muscle contraction and are widely used in treating cardiac disorders. Potassium Channels ION FUNNEL. This side view of a potassium channel reveals its invertedteepee shape embedded in a cell membrane with the extracellular side facing up and the cytoplasmic side down. TIGHT FIT. This view of the potassium channel shows the channel's four identical subunits in different colors. The center of the channel holds a potassium ion (green). ATP-Driven (Active) Transport [Ca2+-ATPase] for removing Ca2+ from cytosol Cytosol Extracellular Selecting a specific cell is a function of a membrane receptor In the case of transferrin iron, the major route of entry is via receptor-mediated endocytosis Receptor-Mediated Endocytosis Structural view of the role of the hemochromatosis protein HFE, a class I major histocompatibility complex (MHC) homolog, in regulating iron uptake by cells. Foreground: two HFE molecules (red and orange ribbons) bound to a homodimeric transferrin receptor (blue ribbons) as seen in the 2.8 angstrom crystal structure of an HFE-transferrin receptor complex. Background: HFE competes with transferrin (green) for binding to transferrin receptor. Localization of calbindin in microvilli and cytosolic vesicles Structural response to calcium in calbindin D9k and calmodulin. Apo calbindin D9k is shown in dark blue. Calciumloaded calbindin D9k is shown in red. Apo N-terminal domain of calmodulin is shown in light blue. Calcium-loaded N-terminal domain of calmodulin is shown in pink. As the picture indicates, the calcium-induced conformational changes are much more pronounced in the calmodulin domains than in calbindin D9k. Intracellular Movement is a function of proteins, low molecular weight ligands and vesicles. 1. The metallochaperones that conduct copper localization 2. Calbindin transfers calcium 3. Glutathione postulated to work with copper Copper Valeria Culotta Undetectable Intracellular Free COPPER: The Requirement of a COPPER Chaperone for Superoxide Dismutase T. D. Rae, 1 P. J. Schmidt, 3 R. A. Pufahl, 1 V. C. Culotta, 3* T. V. O'Halloran 12* The COPPER chaperone for the superoxide dismutase (CCS) gene is necessary for expression of an active, COPPER-bound form of superoxide dismutase (SOD1). In vitro studies demonstrated that purified Cu(I)-CCS protein is is necessary only when the concentration of free COPPER ions ([Cu]free) is strictly limited. Moreover, the physiological requirement for CCS in vivo was readily bypassed by elevated COPPER concentrations. This metallochaperone protein activates the target enzyme through direct insertion of the COPPER cofactor and apparently functions to protect the metal ion from binding to intracellular COPPER scavengers. These results indicate that intracellular [Cu]free is limited to less than one free COPPER ion per cell and suggest that a pool of free COPPER ions is not used in physiological activation of metalloenzymes. Typical Copper Chaperone Hereditary Hemochromatosis (HH) Hepcidin (RR, RE) (Master regulator of iron homeostasis) Positive regulator (+) Intestinal absorption Level Positive regulator (+) HFE (RR) Hemojuvelin (HJV) RR (coded by HFE2 gene) Cellular uptake level Transferrin receptor (RE) Transferrin (RR) Suppresses (-) Ferroportin (RE) Excess Hepcidin = Anemia Defective Hepcidin = Hemochromatosis (HH) RR = regulator RE = regulator target Defective HJV = low Hepcidin (HH) Defective HFE = low Hepcidin (HH) excess transferrin iron uptake