* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download You Light Up My Life - Hawaii Community College
Survey
Document related concepts
Nicotinamide adenine dinucleotide wikipedia , lookup
Electron transport chain wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Transcript
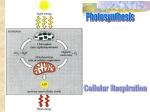
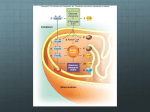
Essential Concepts of Metabolism Chapter 5 Microbiology 130 Metabolism: An Overview MetabolismAnabolism Catabolism- electron transfer OxydationReduction- How do microbes obtain energy? Autotrophs- self feeders, use CO2 to sysnthesis organic compounds - Photoautotrophs- use sunlight for energy - Chemoautotrophs- use inorganics such as sulfides and nitrites for energy Heterotrophs- other feeders, use organic molecules, - Photoheterotrophs-obtain chemical energy from light - Chemoheterotrophs- obtain energy from What Is Energy? Capacity to do work Forms of energy – Potential energy, – Kinetic energy – Chemical energy – What Can Cells Do With Energy? Cells use energy for: – Chemical work – Mechanical work – Electrochemical work One-Way Flow of Energy The sun is life’s primary energy source Producers trap energy from the sun and convert it into chemical bond energy All organisms use the energy stored in the bonds of organic compounds to do work Endergonic Reactions Energy input required glucose, a high energy product Product has more energy than + 6O2 starting substances ENERGY IN 6 low energy starting substances 6 6 6 Exergonic Reactions Energy is released Products have less energy than starting substance glucose, a high energy starting substance + 6O2 ENERGY OUT low energy products 6 6 The Role of ATP Cells “earn” ATP in exergonic reactions Cells “spend” ATP in endergonic base reactions three phosphate groups sugar ATP/ADP Cycle When adenosine triphosphate (ATP) gives up a phosphate group, adenosine diphosphate (ADP) forms ATP can re-form when ADP binds to inorganic phosphate or to a phosphate group that was split from a different molecule Regenerating ATP by this ATP/ADP cycle helps drive most metabolic reactions Participants in Metabolic Reactions Reactants Energy carriers Enzymes Intermediates Cofactors Products Transport Chemical Equilibrium At equilibrium, the energy in the reactants equals that in the products Product and reactant molecules usually differ in energy content Therefore, at equilibrium, the amount of reactant almost never equals the amount of product Chemical Equilibrium Redox Reactions Cells release energy efficiently by electron transfers, or oxidationreduction reactions (“redox” reactions) One molecule gives up electrons (is oxidized) and another gains them (is reduced) Hydrogen atoms are commonly released at the same time, thus becoming H+ Electron Transfer Chains Arrangement of enzymes, coenzymes, at cell membrane As one molecule is oxidized, next is reduced Function in aerobic respiration and photosynthesis Uncontrolled vs. Controlled Energy Release H2 1/2 O2 H2 2H+ Explosive release of energy as heat that cannot be harnessed for cellular work H2O 2e- Energy input splits hydrogen Into protons (H+) and electrons 1/2 O2 Some released energy is harnessed for cellular work (e.g., making ATP) Electrons transferred through electron transfer chain 2e2H+ Spent electrons and free oxygen form water. 1/2 O2 H2 O Metabolic Pathways Defined as enzymemediated sequences of reactions in cells – Biosynthetic (anabolic) – ex: photosynthesis – Degradative (catabolic) – ex: aerobic respiration ENERGY IN ENERGY IN photosynthesis organic compounds, oxygen carbon dioxide, water aerobic respiration ENERGY OUT Enzyme Structure and Function Enzymes are catalytic molecules They speed the rate at which reactions approach equilibrium Four Features of Enzymes 1) Enzymes do not make anything happen that could not happen on its own. They just make it happen much faster. 2) Reactions do not alter or use up enzyme molecules. Four Features of Enzymes 3) The same enzyme usually works for both the forward and reverse reactions. 4) Each type of enzyme recognizes and binds to only certain substrates. Activation Energy For a reaction to occur, an energy barrier must be surmounted Enzymes make the energy barrier smaller activation energy without enzyme starting substance activation energy with enzyme energy released by the reaction products How Catalase Works Induced-Fit Model Substrate molecules are brought together Substrates are oriented in ways that favor reaction Active sites may promote acidbase reactions Active sites may shut out water Factors Influencing Enzyme Activity Temperature pH Salt concentration Allosteric regulators Coenzymes and cofactors Enzyme Helpers Cofactors – Coenzymes NAD+, NADP+, FAD Accept electrons and hydrogen ions; transfer them within cell Derived from vitamins – Metal ions Ferrous iron in cytochromes Allosteric Activation allosteric activator vacant allosteric binding site active site altered, can bind substrate enzyme active site active site cannot bind substrate Allosteric Inhibition allosteric inhibitor allosteric binding site vacant; active site can bind substrate active site altered, can’t bind substrate Feedback Inhibition enzyme 2 enzyme 1 SUBSTRATE enzyme 3 enzyme 4 enzyme 5 A cellular change, caused by a specific activity, shuts down the activity that brought it about END PRODUCT (tryptophan) Effect of Temperature Small increase in temperature increases molecular collisions, reaction rates High temperatures disrupt bonds and destroy the Effect of pH Producing the Universal Currency of Life All energy-releasing pathways – require characteristic starting materials – yield predictable products and byproducts – produce ATP Main Types of Energy-Releasing Pathways Anaerobic pathways Evolved first Don’t require oxygen Start with glycolysis in cytoplasm Completed in cytoplasm Aerobic pathways Evolved later Require oxygen Start with glycolysis in cytoplasm Completed in mitochondria Energy-Releasing Pathways Main Pathways Start with Glycolysis Glycolysis occurs in cytoplasm Reactions are catalyzed by enzymes Glucose (six carbons) 2 Pyruvate (three carbons) The Role of Coenzymes NAD+ and FAD accept electrons and hydrogen from intermediates during the first two stages When reduced, they are NADH and FADH2 In the third stage, these coenzymes deliver the electrons and hydrogen to the transfer chain Anaerobic Pathways Do not use oxygen Produce less ATP than aerobic pathways Two types of fermentation pathways – Alcoholic fermentation – Lactate fermentation Fermentation Pathways Begin with glycolysis Do not break glucose down completely to carbon dioxide and water Yield only the 2 ATP from glycolysis Steps that follow glycolysis serve only to regenerate NAD+ Alcoholic Fermentation glycolysis C6H12O6 2 ATP energy input 2 ADP 2 NAD+ 2 4 NADH ATP energy output 2 pyruvate 2 ATP net ethanol formation 2 H2O 2 CO2 2 acetaldehyde electrons, hydrogen from NADH 2 ethanol Yeasts Single-celled fungi Carry out alcoholic fermentation Saccharomyces cerevisiae – Baker’s yeast – Carbon dioxide makes bread dough rise Saccharomyces ellipsoideus – Used to make beer and wine Lactate Fermentation Carried out by certain bacteria Electron transfer chain is in bacterial plasma membrane Final electron acceptor is compound from environment (such as nitrate), not oxygen ATP yield is low Lactate Fermentation glycolysis C6H12O6 2 ATP energy input 2 NAD+ 2 ADP 2 4 NADH ATP energy output 2 pyruvate 2 ATP net lactate formation electrons, hydrogen from NADH 2 lactate Carbohydrate Breakdown and Storage Glucose is absorbed into blood Pancreas releases insulin Insulin stimulates glucose uptake by cells Cells convert glucose to glucose-6phosphate This traps glucose in cytoplasm where it can be used for glycolysis Glycolysis Occurs in Two Stages Energy-requiring steps – ATP energy activates glucose and its sixcarbon derivatives Energy-releasing steps – The products of the first part are split into three-carbon pyruvate molecules – ATP and NADH form Energy-Requiring Steps ATP ENERGY-REQUIRING STEPS OF GLYCOLYSIS glucose 2 ATP invested ADP P glucose–6–phosphate P fructose–6–phosphate ATP ADP P fructose–1,6–bisphosphate P DHAP Energy-Releasing Steps ENERGY-RELEASING STEPS OF GLYCOLYSIS PGAL PGAL NAD+ Pi NADH P P NADH P 1,3-bisphosphoglycerate ADP NAD+ Pi ATP P 1,3-bisphosphoglycerate ADP ATP substrate-level phosphorylation 2 ATP invested P P 3-phosphoglycerate 3-phosphoglycerate P P 2-phosphoglycerate H2O 2-phosphoglycerate H2O P P PEP PEP ADP ATP ADP ATP substrate-level phosphorylation 2 ATP invested pyruvate pyruvate to second set of reactions Net Energy Yield from Glycolysis Energy requiring steps: 2 ATP invested Energy releasing steps: 2 NADH formed 4 ATP formed Net yield is 2 ATP and 2 NADH Overview of Aerobic Respiration Overview of Aerobic Respiration C6H1206 + 6O2 glucose oxygen 6CO2 + 6H20 carbon dioxide water Overview of Aerobic Respiration glucose cytoplasm 2 ATP ATP GLYCOLYSIS energy input to start reactions e- + H+ (2 ATP net) 2 pyruvate 2 NADH mitochondrion 2 NADH 8 NADH 2 FADH2 e- e- + H+ 2 CO2 e- + H+ 4 CO2 e- + H+ Krebs Cycle 2 ELECTRON TRANSPORT PHOSPHORYLATION H+ 32 ATP ATP water e- + oxygen TYPICAL ENERGY YIELD: 36 ATP Second-Stage Reactions Occur in the mitochondria Pyruvate is broken down to carbon dioxide More ATP is formed More coenzymes are reduced Two Parts of Second Stage Preparatory reactions – Pyruvate is oxidized into two-carbon acetyl units and carbon dioxide – NAD+ is reduced Krebs cycle – The acetyl units are oxidized to carbon dioxide – NAD+ and FAD are reduced Preparatory Reactions pyruvate + coenzyme A + NAD+ acetyl-CoA + NADH + CO2 One of the carbons from pyruvate is released in CO2 Two carbons are attached to coenzyme A and continue on to the Krebs cycle Using Glycogen When blood levels of glucose decline, pancreas releases glucagon Glucagon stimulates liver cells to convert glycogen back to glucose and to release it to the blood (Muscle cells do not release their stored glycogen) Energy Reserves Glycogen makes up only about 1 percent of the body’s energy reserves Proteins make up 21 percent of energy reserves Fat makes up the bulk of reserves (78 percent) Energy from Fats Most stored fats are triglycerides Triglycerides are broken down to glycerol and fatty acids Glycerol is converted to PGAL, an intermediate of glycolysis Fatty acids are broken down and converted to acetyl-CoA, which enters Krebs cycle Energy from Proteins Proteins are broken down to amino acids Amino acids are broken apart Amino group is removed, ammonia forms, is converted to urea and excreted Carbon backbones can enter the Krebs cycle or its preparatory reactions Reaction Sites FOOD fats fatty acids glycogen glycerol complex carbohydrates proteins simple sugars (e.g., glucose) amino acids NH3 glucose-6phosphate urea carbon backbones PGAL 2 glycolysis ATP 4 ATP (2 ATP net) NADH pyruvate Acetyl-CoA NADH NADH, FADH2 CO2 Krebs Cycle 2 ATP CO2 e– ATP ATP ATP H+ e– + oxygen many ATP fats Evolution of Metabolic Pathways When life originated, atmosphere had little oxygen Earliest organisms used anaerobic pathways Later, noncyclic pathway of photosynthesis increased atmospheric oxygen Cells arose that used oxygen as final acceptor in electron transfer Aerobic Respiration Reactants – Sugar – Oxygen Products – Carbon dioxide – Water Photosynthesis Reactants – Carbon dioxide – Water Products – Sugar – Oxygen Processes Are Linked Life Is System of Prolonging Order Powered by energy inputs from sun, life continues onward through reproduction Following instructions in DNA, energy and materials can be organized, generation after generation With death, molecules are released and may be cycled as raw material for next generation