* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Press release as pdf
Survey
Document related concepts
Chemical synapse wikipedia , lookup
Cell membrane wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Endomembrane system wikipedia , lookup
NMDA receptor wikipedia , lookup
Killer-cell immunoglobulin-like receptor wikipedia , lookup
List of types of proteins wikipedia , lookup
Purinergic signalling wikipedia , lookup
Leukotriene B4 receptor 2 wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Transcript
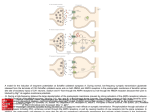
PRESS RELEASE An important step towards highly effective drugs Microscale thermophoresis paves the way for a new kind of drug-screening assay based on G protein-coupled receptors (GPCRs) Munich, May 12, 2011 - Receptor proteins located within the cell membrane serve to convey molecules or information into the interior. Because numerous drugs function by binding to such receptors, their interactions play important roles in the fight against various diseases. For example, the coupling can activate a signal chain which in the end specifically changes the metabolism of malignant cells. A collaboration between biophysicists from the LMU and researchers at the Massachusetts Institute of Technology (MIT) in Boston has found a very elegant and potentially revolutionary new way to screen for drug binding to membrane receptors. The Munich part of the project was led by scientists of the Nanosystems Initiative Munich (NIM) and of NanoTemper, a commercial spin-off of the LMU. The new approach will allow screening of the most common class of receptors (G protein-coupled receptors, GPCRs) for the binding of new drugs directly in solution. The study appears online in the recent Proceedings of the National Academy of Sciences of America (PNAS). Membrane receptors coordinate communications between cells – whether between singlecelled organisms or the cells of a complex organism such as the human body. The huge receptor proteins traverse the cell membrane from the exterior surface to the inside. When a messenger molecule – or a pharmaceutical - successfully binds at the external surface, the protein conformation changes. This, in turn, activates a signal chain inside the cell. As potential drug targets, receptor proteins are highly in demand for testing the efficacy of new pharmaceuticals. Of special interest are the G protein-coupled receptors (GPCRs) because a wide range of diseases can be successfully treated with GPCR-targeting drugs. But there are two major challenges: First, receptor proteins are very fragile and normally need to be embedded in a membrane. Second, drug candidates are typically much smaller than their potential receptors. Therefore it is nearly impossible to test the binding directly by conventional methods which depend on measuring changes in mass or size. Alternatives, such as indirect cell culture experiments, take much more time and consume more material. The researchers from Boston and Munich combined two innovative techniques to overcome these barriers. The MIT group specializes in putting G protein-coupled receptors (GPCRs) into membrane-like structures made from artificial peptides. In this way the artificially produced receptors remain correctly folded and soluble. Nanosystems Initiative Munich (NIM) Dr. Birgit Gebauer (Presse- und Öffentlichkeitsarbeit) Schellingstraße 4, D-80799 München Tel.: +49 (89) 2180 5091 Fax: +49 (89) 2180 5649 Mail: [email protected] The biophysicists of the LMU used microscale temperature gradients to detect drug binding. This method is based on the fact that molecules - as for example the receptors - in solution move along a temperature gradient in a characteristic way. The scientists established a microscopic temperature gradient by localized laser heating of a test tube containing about 1 microliter sample solution. They compared the movement of the pure receptor with the molecule’s behavior after addition of a test drug. If the drug binds to the receptor molecule, the characteristics of its movement change. Not only is the setup of the so-called microscale thermophoresis (MST) binding assay very robust. The method is so sensitive that small, binding-induced changes in the conformation or shape of a GPCR can be detected using very small amounts of protein and test sample. By varying the amount of drug, the efficacy of the binding can be determined quantitatively. These results document the successful combination of soluble GPC receptors with the microscale thermophoresis (MST) recently developed by the NanoTemper startup company. The new approach has great potential to become a simple and rapid standard assay for pharmaceutical and basic research (NIM). Publication: Peptide surfactants for cell-free production of functional G protein-coupled receptors. Xiaoqiang Wang, Karolina Corin, Philipp Baaske, Christoph J. Wienken, Moran JerabekWillemsen, Stefan Duhr, Dieter Braun, Shuguang Zhang. Proceedings of the National Academy of Sciences of America (PNAS), Published online May 9, 2011. Contact: Prof. Dieter Braun Systems Biophysics, Ludwig-Maximilians-Universität München Amalienstrasse 54 80799 München Tel: 089-2180-2317 Mail: [email protected] Dr. Philipp Baaske NanoTemper Technologies GmbH Amalienstrasse 54 80799 München Tel: 089-2180 2833 Mail: [email protected] Nanosystems Initiative Munich (NIM) Dr. Birgit Gebauer (Presse- und Öffentlichkeitsarbeit) Schellingstraße 4, D-80799 München Tel.: +49 (89) 2180 5091 Fax: +49 (89) 2180 5649 Mail: [email protected]