* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Grant MacEwan College - Faculty Web Pages
Bottromycin wikipedia , lookup
Kinetic resolution wikipedia , lookup
Aromaticity wikipedia , lookup
Homoaromaticity wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromatization wikipedia , lookup
Discodermolide wikipedia , lookup
Ene reaction wikipedia , lookup
Elias James Corey wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
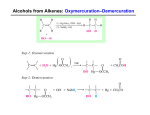
MacEwan University Winter 2014 CHEM 263 (41) Organic Chemistry II Instructor: Office: Dr. Manzar Saberi 5- 138 G, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday 11:00-12:30 CCC Room 6-291 Office Hours: Tuesday, Thursday, 9:30-11:10, and Wednesday 11:00-12:00 Web address: http://academic.macewan.ca/saberim Textbook: Organic Chemistry by T. W. Graham Solomons and Craig B. Fryhle, 11th edition Grade Distribution: 1st Term- Thursday, February 6 (80 min) 22.5 % 2nd Term- Thursday, March 13 (80 min) 22.5% Final Examination- April 17, AM (3.0 hours) 30 % Laboratory Examination- April 11 (6:00-7:30 PM) 25% Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functional groups in natural products is provided. The application of spectroscopic methods for structural determination in simple molecules is discussed. Prerequisite: Minimum grade of C- in CHEM 161 or 261 The Faculty of Arts and Science strictly adhere to the notion of prerequisites, and University staff conducts prerequisite checks throughout the term. If it is discovered that you do not have the appropriate prerequisite for this course, you will be withdrawn by the Registrar’s Office. Deciding to remain in the course without the prerequisite may result in a significant financial penalty because you will be responsible for any tuition costs associated with the course up to the date of the withdrawal. Courses transferred to Grant MacEwan University from another post-secondary institution will not be applied to your student record until a transfer credit assessment has been completed. If you believe you have the proper external prerequisite please consult with an advisor in the program office (6-211). Laboratory: Laboratory classes begin the second week of the term. The laboratory component is compulsory for credit in CHEM 263; attendance is mandatory and no make-up labs are available. If you know that you will be unable to attend a scheduled laboratory period, it is your responsibility to inform your laboratory instructor at least one week prior so that you can complete the experiment in another laboratory session. If a laboratory period is missed for a valid reason, the experiment may not be counted towards the final laboratory grade. In all other cases, a mark of zero will be assigned. Students who miss more than two labs will not receive credit for the laboratory component. Students must pass the laboratory component (minimum 50 %) and at least 70% of the experiments in order to pass the course. University Information Grading: MacEwan University adheres to the Alberta Common Grading Scheme, which is a letter grade system. While instructors may use percentages to aid in their grade development, only the letter grade will appear on transcript. A+ A A– B+ 100 > 95 95 > 90 90 > 85 85 > 80 B B– C+ C 80 > 75 > 70 > 65 > 75 70 65 60 C– D+ D F 60 > 55 55 > 50 50 > 45 45 > Unofficial FINAL grades (lecture and laboratory) will be posted on my website approximately one week after each respective final exam at http://academic.macewan.ca/saberim A minimum grade of C- is required to receive transfer credit or to satisfy a prerequisite for higher-level course. Important Dates: 06 Jan 13 Jan 13 Jan 17 – 21 Feb 21- March 14 – 26 Apr First day of classes Laboratory classes begin Last day for program changes (drop/add courses) Reading Week (no classes or laboratories) Last day to withdraw from courses (without academic penalty) Examination period Students are expected to be aware of their academic responsibilities as outlined in MacEwan University Policy E3101: Student Rights and Responsibilities (found here). 1. Academic Integrity: All forms of student dishonesty are considered unacceptable. MacEwan University Policy C1000: Academic Integrity (found here) promotes honesty, fairness, respect, trust, and responsibility in all academic work. According to the policy, “Academic dishonesty involves participating in acts by which a person fraudulently gains or intentionally attempts to gain an unfair academic advantage thereby compromising the integrity of the academic process”. All incidents of academic dishonesty are reported and recorded by the Academic Integrity Office. The penalties and sanctions for academic dishonesty can include the following: a mark reduction up to zero on a piece of academic work, a grade reduction up to an F in the course, and suspension or expulsion (with transcript notation) from the University. Please see the academic integrity policy for more details. You are responsible for understanding what constitutes academic dishonesty. 2. Registration Status: You are responsible for your registration status at the University. Program advisors (Rm 6-211) may assist you with the process of registration, including adding or dropping of courses, but it is your responsibility to verify that these changes have been officially completed. This verification can be done at any time using myStudentSystem. You should check your official registration status before the last date to officially withdraw from the course. 3. Withdrawing From The Course: If you stop attending class you must complete a Course Drop Form, have it signed by a Program Advisor (Rm 6-211), and submit it to the Registrar’s Office by the last day to withdraw as provided in the Academic Schedule in the Academic Calendar. Failure to officially withdraw will result in a grade being assigned based on course work completed. Late withdrawals are only allowed in exceptional circumstances. 4. Exams: Your student photo I.D. is required at exams. It is at the discretion of the instructor whether you will be allowed to write the exam if you arrive over 15 minutes after the exam has begun. You must remain in the exam room for at least 20 minutes from the time it commenced. Only calculators approved for use by the instructor may be used during examinations, which include any laboratory and lecture quizzes. Any devices capable of external communication, such as cell phones, iPods and blue tooth enabled devices, cannot be used for exams of any type. Permission to use the washroom during exams is at the discretion of the instructor and may require accompaniment. 5. Missed Term Exams: If you miss a term examination you must provide the instructor with an explanation within 24 hours or a mark of zero may be given. Notification may be provided through email, voice mail, or direct contact with the instructor. Official documentation as to why the examination was missed will be needed to assess whether your absence will be excused or not. If your absence is excused the weight of this examination will be added to the weight of the final examination in the course. Medical excuses must include the date you were examined, the specific dates for the period of the illness, a clear statement indicating that the severity of the illness prevented you from attending school or work, and the signature of the examining physician (a signature by office staff on behalf of the physician is not acceptable). Medical notes obtained subsequent to the date of the examination are generally not accepted. A mark of zero will be given if the instructor considers the excuse inappropriate or inadequately substantiated. 6. Deferred Final Exam: A deferred examination will be granted if a student misses the final lecture examination for reasons considered by the Bachelor of Science Program to be unavoidable (deferred examinations do not apply to term or laboratory examinations). An application for a deferred examination must be provided to the Faculty of Art and Science Program (Rm 6-211) no later than two business days after the date of the missed final examination. Application forms are available from the Program Services office, and must be submitted with appropriate documentation. Students should advise the instructor prior to the examination if they know beforehand that they will be unable to attend the scheduled examination time. Deferred examinations are granted by the Chair, Bachelor of Science Program, not by the course instructor. If you have any questions about the process please contact Program Services (780 497 4520 or [email protected]). For further information please refer to MacEwan University Policy C2005: Final Assessment and Policy C2020: Grading. Note that C2005 states: only a compelling situation such as serious illness, hospitalization, domestic affliction or religious observance will be considered a valid reason for a student to receive approval for deferral of a final assessment activity and personal vacations are not a sufficient reason. 7. Late Assignments (including laboratory assignments): As due dates for assignments are known well in advance, medical and other excuses are generally not accepted as a reason for submitting late assignments. 8. Cell Phones: All cell phones are to be turned off during class and exam periods (except under exceptional circumstances in which approval has been given by the instructor). 9. Students with Disabilities: Students with disabilities who may have special requirements in this course are advised to discuss their needs with Services to Students with Disabilities located in the Student Resource Centre. You should advise the course instructor(s) of any special needs that are identified. See Policy E3400 Students with Disabilities (found here). 10. Student Appeals: The University has a policy regarding Student Appeals (E3103, found here). You should access this policy to become aware of the deadlines and guidelines that need to be followed if you are appealing a grade or other University assessment. 11. MyMacEwan.ca Email: All students are given a <name>@mymacewan.ca email address. This email address is available to the course instructor who may distribute relevant course information or announcements via email. The Bachelor of Science Program regularly communicates with students via email. Check your mymacewan.ca email regularly or forward it to an email address you check regularly. If you use email to communicate with your instructor, you must use your mymacewan.ca account. This is to protect your privacy; if a non-mymacewan.ca account is used, there is no way for an instructor to verify the identity of the sender. Disclaimer: The information in this course outline is subject to change. Any changes will be announced in class or, if applicable, in the laboratory. Chemistry 263 Course Outline 7. Hydrogenation of Alkenes - Syn and anti- addition - Hyrdogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-Alkenes -Anti addition of hydrogen: Synthesis of trans-Alkenes Text Sections: 7.12-7.14. 8. Alkenes and Alkynes II: Addition reactions Addition reactions of alkenes How to understand addition to alkenes Electrophilic addition of hydrogen halides to alkenes: Mechanism and Markovnikov’s Rule Theoretical Explanation of Markovnikov’s Rule - General statement of Markovnikov’s Rule - regioselective reactions - exception to Markovnikov’s rule Stereochemistry of the ionic addition to an alkene Addition of water to alkenes: Acid-catalyzed hydration - mechanism - rearrangements Alkohols from alkenes through oxymercuration-demercuration: Markovnikov’s addition - Regioselectivity of oxymercuration-demercuration - Mechanism of oxymercuration Alcohols from alkenes through hydroboration-oxidation:Anti-Markovnikov syn hydration Hydroboration: Synthesis of alkylboranes - mechanism of hydroboration - stereochemistry of hydroboration Oxidation and hydrolysis of alkylboranes - regiochemistry and stereochemistry of alkylborane oxidation and hydrolysis Summary of alkene hydration methods Electrophilic addition of bromine and chlorine to alkenes - mechanism of halogen addition - stereospecific reactions Halohydrin formation mechanism for the reaction Oxidation of alkenes: Syn 1,2-dihydroxylation - mechanism for syn dihydroxylation of alkenes Oxidative cleavage of alkenes - cleavage with hot basic potassium permanganate - cleavage with ozone Electrophilic Addition of bromine and chlorine to alkynes Addition of hydrogen halides to alkynes Oxidative cleavage of aklynes How to plan a synthesis: some approach and examples - retrosynthetic analysis - disconnections, synthons, and synthetic equivalents - stereochemical considerations Text Sections: 8.1- 8.9, 8.11- 8.13, 8-15-8.20 9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - chemical shift - integration of signal area - coupling (signal splitting) How to interpret proton NMR spectra Nuclear Spin: The origin of the signal Detecting the signal: Fourier transform NMR spectrometers Shielding and deshielding of protons The chemical shift - PPM and the δ scale Chemical shift equivalent and nonequivalent protons - Homotopic and heterotopic atoms - Enantiotopic and diastereotopic hydrogen atoms Signal slitting: Spin-spin coupling - Vicinal coupling - Splitting tree diagrams and the origin of signal splitting - Coupling constants-recognizing splitting patterns - The dependence of coupling constants on dihedral angle - Complicating features - Analysis of complex integrations Proton NMR spectra and rate processes Text Sections: 9.1-9.9. 10. Radical Reactions Introduction: How radicals forms and how they react - Production of radicals - Reactions of radicals Homolytic bond dissociation energy (∆Ho) - How to use hemolytic bond dissociation energies to calculate heat of reaction - How to use hemolytic bond dissociation energies to determine the relative stabilities of radical Reactions of alkenes with halogens - Multiple halogen substitution - Lake of chlorine selectivity Chlorination of methane: Mechanism of reaction - Activation energies - Reaction of methane with other halogens Halogenation of higher alkanes - Selectivity of bromine The geometry of alkyl radicals Reactions that generate tetrahedral chirality centers - Generation of a second chirality center in a radical halogenations Radical addition to alkenes: The anti-Markovnikov addition of hydrogen bromide - Summary of Markovnikov versus anti-Markovnikov addition of HBr to alkenes. Text Sections: 10.1-10.7, 10.9-10.10. 11. Alcohols and Ethers Structure and Nomenclature - nomenclature of alcohols - nomenclature of ethers Physical Properties of Alcohols and Ethers Synthesis of Alcohols from Alkenes Reactions of Alcohols Alcohols as Acids Conversion of Alcohols into Alkyl Halides Alkyl Halides from the Reaction of Alcohols with Hydrogen Halides - mechanism of the reactions of alcohols with HX Alkyl halides from the reaction of alcohols with PBr3 or SOCl2 Tosylate, mesylates, and triflates: Leaving group derivatives of alcohols Synthesis of Ethers - ethers by intermolecular dehydration of alcohols - the Williamson Ether Synthesis of ethers - synthesis of ethers by Alkoxymercuration-demercuration. Reactions of Ethers - ether cleavage Epoxides - synthesis of epoxides: epoxidation - stereochemistry of epoxidation Reactions of epoxides - acid-catalyzed ring opening of an epoxide - base-catalyzed ring opening of an epoxide Anti 1,2-dihydoxylation of Alkenes via Epoxides Summary of reactions of alkenes, alcohols, and ethers - how alkenes can be used in synthesis Text Sections: 11.1 –11.2, 11.4-11.15. (no 11.11 D, E and 11.14 A) . 12. Alcohols from Carbonyl Compounds. Oxidation-reduction and Organometallic compounds Structure of the Carbonyl group - reactions of Carbonyl Compounds with Nucleophiles Oxidation-reduction Reactions in Organic Chemistry - oxidation states in organic chemistry Alcohols by Reduction of Carbonyl Compounds - lithium aluminum hydride - sodium borohydride - overall summary of LiAlH4 and NABH4 reactivity Oxidation of alcohols - oxidation of primary alcohols to aldehyde; pyridinium chlorochromate (PCC) - oxidation of primary alcohols to carboxylic acids - oxidation of secondary alcohols to ketones - mechanism of chromate oxidations - a chemical test for primary and secondary alcohols - spectroscopic evidence for alcohols Organometallic Compounds Preparation of organolithium and organomagnesium compounds - organolithium compounds - Grignard reagents Reactions of organolithium and organomagnesium compounds - reactions with compounds containing acidic hydrogen atoms - reactions of Grignard reagents with epoxides - reactions of Grignard reagents with carbonyl compounds Alcohols from Grignard Reagents How to plan a Grignard synthesis Restrictions on the use of Grignard reagents The use of lithium reagents The use of sodium alkynides Text Sections: 12.1 – 12.3, no 12.3D) 12.4(no 12.4 B)-12.8. 14. Aromatic Compounds Nomenclature of Benzene Derivatives Text Sections: 14.2 15. Reactions of aromatic Compounds Electrophilic Aromatic Substitution Reactions A General Mechanism for Electrophilic aromatic substitution Reactions of Benzenes - halogenation - nitration - sulfonation - Fridel-Crafts alkylation - Friedel-Crafts Acylation - Limitation of Friedel-Craft reactions Synthetic Applications of Friedel-Crafts Acylations: The Clemmensen Reduction Substituents can affect both the reactivity of the ring and the orientation of the incoming group - how do substituents affect reactivity? - ortho-para directing groups and meta-directing groups - electron-donating and electron-withdrawing substituents - ortho-para directors - deactivating groups: Meta directors - halo substituents: Deactivating ortho-para directors - classification of substituents How substituents affect electrophilic aromatic substitution: A closer look - reactivity: The effect of electron-releasing and electron-withdrawing groups - inductive and resonance effects: Theory of orientation - meta-directing groups - ortho-para-directing groups - ortho-para direction and reactivity of alkylbenzene Reactions of the Side Chain of Alkylbenzenes: - benzylic radicals and cations - benzylic halogenations of the side chain - oxidation of the side chain Alkenylbenzenes: - stability of conjugated alkenylbenzenes - addition reaction to the double bond of alkenylbenzenes Synthetic Applications - use of protecting and blocking groups - orientation in disubstituted benzenes Allylic and Benzylic Halides in Nucleophilic Substitution Reactions Text Sections: 15.1- 15.9, (no 15-9 B), 15.10-15.13, (no 15.13 D), 15.14-15.15. 16. Aldehydes and Ketones. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Physical Properties Synthesis of aldehydes: - aldehyde by oxidation of 1o alcohols - aldehydes by ozonolysis of alkene - aldehydes by reduction of acyl chlorides, esters, and nitriles Synthesis of ketones: - ketones from alkenes, arenes, and 2o alcohols - ketones from nitriles Nucleophilic Addition to the Carbon-oxygen Double Bond - relative reactivity of aldehydes vesus ketones - reversibility of nucleophilic addition to the carbon-oxygen double bond - addition products can undergo further reactions The addition of alcohols: Hemiacetals and acetals - hemiacetals - acetals - acetals are used as protecting groups The addition of primary and secondary amines imines - enamines The addition of hydrogen cyanide: Cyanohydrins The addition of Ylides: The Wittig reaction - how to plan a Wittig synthesis Oxidation of aldehydes Tollen’s test (silver mirror test) Spectroscopic properties of aldehydes and ketones. 1H NMR, IR spectroscopy. Summary of aldehyde and ketone addition reactions Text Sections: 16.1-16.7 (no 16.7 D), 16.8 (no 16.8 B & C), 16.9-16.10(no 16.10 B), 16.11, 16.13-16.14. 17. Carboxylic Acids and their Derivatives Introduction Nomenclature and physical Properties - carboxylic acids - carboxylate salts - acidity of carboxylic acids - dicarboxylic acids Carboxylic acid Derivatives: - esters - carboxylic anhydrides - acyl chlorides - amides - nitriles Spectroscopic Properties of Acyl Compounds - 1H NMR - IR spectra Preparation of Carboxylic acids by: oxidation of alkenes oxidation of aldehydes and primary alcohols oxidation of alkylbenzene - hydrolysis of cyanohydrins and other nitriles - carbonation of Grignard reagents Acyl substitution: Nucleophilic Addition – Elimination at the Acyl carbon - relative reactivity of acyl compounds Acyl chlorides - synthesis of acyl chloride using thionyl chloride Synthesis of esters: Fischer esterification - mechanism of the esterification reaction Spectroscopic analysis of Acids 1H NMR, IR spectroscopy Text Sections: 17.1 – 17.3 (no17.3 (4), 17.4-17.5 A, 17.7 A. 20. Amines Nomenclature of Amines - arlyamines Physical Properties and structure of amines - basicity of amine : Amine salts - basicity of arlyamines - amines versus amides - solubility of amines in aqueous acid Preparation of amines - through nucleophilic substitution reactions - the Gabriel synthesis - preparation of aromatic amines through reduction of nitro compounds - preparation of primary, secondary, and tertiary amines through reductive amination - preparation of primary, secondary, or tertiary amines through reduction of nitriles, oximes, and amides Spectroscopic analysis of Amines 1H NMR, IR spectroscopy Text Sections: 20.1(A) –20.3(A-E, no B & F), 20.4, 20.11 Organic Chemistry by T. W. Graham Solomons and Craig B. Fryhle, and Scott A. Synder 11th edition