* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glutamate synthase and nitrogen
Magnesium transporter wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
Point mutation wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Gene regulatory network wikipedia , lookup
Gene expression wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Metalloprotein wikipedia , lookup
Gene expression profiling wikipedia , lookup
Plant breeding wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Expression vector wikipedia , lookup
Plant nutrition wikipedia , lookup
Biochemistry wikipedia , lookup
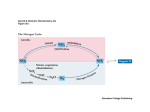
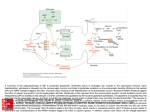
trends in plant science reviews Glutamate synthase and nitrogen assimilation Stephen J. Temple, Carroll P. Vance and J. Stephen Gantt The assimilation of ammonia by a wide variety of organisms is the primary route for the introduction of nitrogen into the biosphere. The assimilatory enzymes glutamine synthetase and glutamate synthase catalyze reactions that convert a-ketoglutarate and ammonia to glutamate, which is then used in a wide variety of biosynthetic reactions. These enzymes also play a major role in the reassimilation of ammonia derived from photorespiration in C3 plants. Recent biochemical, molecular and genetic studies are leading to a better understanding of the factors that determine the activity and function of glutamate synthase. N itrogen is often the major limiting nutrient for plant growth. Most plants obtain their nitrogen from soil nitrate, which is largely derived from either fertilizers or the mineralization of indigenous organic matter. The nitrate is converted to NH4+ by the sequential reductive action of the plant enzymes nitrate reductase and nitrite reductase. Some plants, most notably many legumes, can also obtain their nitrogen from atmospheric N2. These legumes form a symbiotic association with rhizobia, which are able to reduce N2 to NH4+ by the action of nitrogenase (Fig. 1). Ammonia is then transferred from the microbe to the plant. Plants also produce significant amounts of NH4+ from photorespiration, phenylpropanoid biosynthesis and amino acid catabolism. Consequently, the reduced form of nitrogen ultimately available to higher plants for assimilation is NH4+, irrespective of the primary nitrogen source1. However, because NH4+ is toxic to plant cells, because of its ability to uncouple respiration at low conFig. 1. Diagram showing nitrogen assimilation in legume root nodules and the cellular locentrations, it must be rapidly assimilated cation of the enzymes involved. In the diagram, glutamine, asparagine and ureides derived into non-toxic organic compounds. This from purines are the primary nitrogenous compounds exported to other cells and provided necessitates strict regulatory control of the for transport throughout the plant. Photosynthate provides the carbon skeletons for amino nitrogen-assimilation pathway. acid biosynthesis via glycolysis and the tricarboxylic acid cycle. Substantial additional carPrior to 1970 it was generally assumed bon can also be derived from non-photosynthetic CO2 fixation via phosphoenolpyruvate. that ammonia was assimilated by the direct The enzymes involved are: AAT, aspartate aminotransferase; AS, asparagine synthase; GOGAT, glutamate synthase; GS, glutamine synthetase; MDH, malate dehydrogenase; Nit, amination of a-ketoglutarate to produce nitrogenase; PEPC, phosphoenolpyruvate carboxylase. glutamate in a single reaction catalyzed by glutamate dehydrogenase. However, the plant enzymeÕs high Km for NH4+ (>1 mM) suggested that glutamate dehydrogenase did not have a major role route of nitrogen assimilation in plants (Fig. 2). The synthesized in nitrogen assimilation2. Subsequent studies using radiolabeled glutamate can be used either to replenish the pool of glutamate for (13N) and stable (15N) nitrogen isotopes, enzyme inhibitors and subsequent glutamine synthetase catalysis or to donate its amino mutants of plant nitrogen metabolism indicated that the primary group to form other nitrogen-containing compounds. One imporassimilation of NH4+ into amino acids occurs via the joint action tant fate of glutamate and glutamine is the synthesis of aspartate of glutamine synthetase and glutamate synthase [also termed glu- and asparagine, produced in reactions catalyzed by aspartate tamate 2-oxoglutarate aminotransferase (GOGAT)] (Fig. 1)3. The aminotransferase and asparagine synthetase (Fig. 1). These amino reaction catalyzed by glutamine synthetase involves the ATP- acids are important nitrogen-transport compounds in many plants. dependent amination of glutamate to yield glutamine. GOGAT Carbon skeletons required for these initial NH4+-assimilatory then catalyzes the transfer of the amide group from glutamine reactions are provided by a-ketoglutarate and oxaloacetate1. The to a-ketoglutarate to yield two molecules of glutamate. These requirement for and metabolism of these tricarboxylic acid cycle two reactions, collectively referred to as the glutamine syn- intermediates tightly link nitrogen assimilation and carbon thetase/GOGAT cycle, are now generally agreed to be the primary metabolism. Copyright © 1998 Elsevier Science Ltd. All rights reserved. 1360 - 1385/98/$19.00 PII: S1360-1385(97)01159-X February 1998, Vol. 3, No. 2 51 trends in plant science reviews range from 7.5 to 8.5; and apparent Km values for NADH, glutamine and aketoglutarate of 4Ð13, 400Ð1000 and 39Ð 960 mM, respectively6. In root nodules, NH4+ is exported from the nitrogen-fixing bacteroids to the host-plant cytoplasm, where it is rapidly assimilated via NADHGOGAT and cytosolic glutamine synthetase into amino acids (Fig. 1). There is negligible NADH-GOGAT mRNA, enzyme activity or immunoreactive protein in alfalfa leaves and roots1. In bean nodules, NADH-GOGAT appears to occur as two isoforms (I and II), with the observed increase in GOGAT activity during nodule development resulting primarily from an increase in activity of isozyme II (Ref. 12). The expression patterns of the genes encoding cytosolic glutamine synthetase and NADH-GOGAT appear to be coordinated in non-legumes, where the proteins function together in processes such as the primary assimilation of NH4+ derived from soil NO32, the reassimilation of NH4+ released during amino acid catabolism, Fig. 2. The glutamate synthase cycle. The first reaction is catalyzed by glutamine synthetase and/or the reassimilation of NH4+ released L-glutamate: ammonia ligase (ADP-forming) (EC 6.3.1.2). The second reaction is catalyzed + during seed germination3,8. In a study with by glutamate synthase (GOGAT), which can exist in two forms: L-glutamate: NAD oxidorice, NADH-GOGAT protein and activity reductase (transaminating) (EC 1.4.1.14); and L-glutamate: ferredoxin oxidoreductase increased four- and sixfold, respectively, in (transaminating) (EC 1.4.7.1). Reaction 3 shows the net metabolic balance and energy costs the apical spikelets during the first 15 d of the glutamine synthetase/GOGAT cycle. after flowering; the levels reached a maximum when seed storage protein accumulation was initiated13. The majority of the Biochemistry and enzymology of glutamate synthase NADH-GOGAT protein was found to be associated with the In higher plants, GOGAT occurs as two distinct isoforms, NADH- young grain tissue. Although changes in Fd-GOGAT activity parGOGAT (EC 1.4.1.14) and ferredoxin-dependent GOGAT (Fd- alleled the changes in NADH-GOGAT activity, the relative abunGOGAT) (EC 1.2.7.1); these differ in molecular mass, subunit dance of NADH-GOGAT was about threefold higher than that of composition, enzyme kinetics, antigenic and reductant specificity, Fd-GOGAT (Ref. 13). These results suggest that in rice NADHand metabolic function4,5. Fd-GOGAT is an ironÐsulphur flavo- GOGAT is responsible for the synthesis of glutamate from the protein with a subunit molecular mass of 130Ð180 kDa that is gen- glutamine that is transported from senescing tissues to the erally considered to function as a monomer. The enzyme has a pH spikelets. optimum of 6.9Ð7.5 and apparent Km values for ferredoxin, glutamine and a-ketoglutarate of 2Ð6, 100Ð1000 and 7Ð70 mM, Evolutionary and structural relationships among diverse respectively6. In combination with the plastid-localized isoform of glutamate synthase proteins glutamine synthetase, Fd-GOGAT catalyzes the assimilation of GOGAT is found in all types of organisms, and its amino acid NH4+ derived from both the light-dependent reduction of NO32 sequence is remarkably well conserved. To examine the evoluand the NH4+ generated during photorespiration5. Maize roots tionary relationships among the eubacterial and eukaryotic contain an Fd-GOGAT isoform that is immunologically distinct GOGAT proteins, a phylogenetic tree can be constructed based on from the enzyme found in leaves, suggesting that the two forms the amino acid sequences of regions common to all eubacterial are encoded by distinct genes. The root isoform has been impli- and eukaryotic GOGAT proteins (Fig. 3). With the exception of cated in the assimilation of NH4+ derived from soil NO32 (Ref. 7). the Synechocystis sp. gltB gene product, all of the Fd-GOGAT Recent studies indicate that Arabidopsis also contains two distinct proteins cluster together. This group contains Fd-GOGATs from and apparently functional Fd-GOGAT genes (GLU1 and GLU2). higher plants (maize, rice and Arabidopsis), two red alga species The GLU1 gene is expressed predominantly in leaves, and GLU2 (Porphyra purpurea and Antithamnion sp.) and two cyanobacteria expression is more abundant in roots8,9. (Synechocystis sp. and Plectonema boryanum). This analysis Although NADH-GOGAT, like Fd-GOGAT, is also an shows that eukaryotic Fd-GOGAT is closely related to bacterial ironÐsulphur flavoprotein, this enzyme is found primarily in non- Fd-GOGATs, and suggests that the genes encoding these enzymes green tissues. NADH-GOGAT has been purified and character- are derived from the eubacterial precursors of chloroplasts. These ized from legume root nodules and rice cell cultures4,10,11. In data are consistent with an endosymbiotic origin of plastids. Addinitrogen-fixing legume nodules, NADH-GOGAT activity has tional support for this conclusion comes from the finding that the been found to increase markedly during nodule development, and Fd-GOGAT gene is found in the plastid genomes of red algae14. this activity is associated with a single form of the enzyme4. In Higher plant GOGAT genes are all located in the nuclear genome, higher plants, NADH-GOGAT exists as monomers with a native into which they were presumably transferred from the endosymsubunit mass of approximately 225Ð230 kDa; has a pH-optimum biont genome15. The phylogenetic analysis also suggests that the 52 February 1998, Vol. 3, No. 2 trends in plant science reviews gltB gene, which has been found in all cyanobacteria examined, is generally more similar to eukaryotic and eubacterial NAD(P)H-GOGATs than to Fd-GOGATs, and did not functionally replace the NADH-GOGAT genes found in higher plants. The structural relationships among the various GOGAT proteins are quite diverse16 (Fig. 4). In general, the enzyme found in non-photosynthetic eubacteria uses NADPH as a cofactor and is composed of two subunits. The large subunit, encoded by gltB, contains a PurF-type amidotransferase domain near its N-terminus and a 3Fe/4S center. The small subunit may contain additional ironÐsulphur centers and an NADPH-binding region17. In eukaryotes, which contain a single ironÐsulphur center18, sequences similar to both of these subunits, separated by a short hydrophilic region, are found in the single NADH-GOGAT polypeptide. Fd-GOGAT is structurally similar only to the large subunit of the eubacterial NADPH-GOGAT Fig. 3. An unrooted phylogenetic tree of NAD(P)H- and Fd-glutamate synthase (GOGAT) protein. These data suggest that the proteins. The neighbor-joining method32 found in the CLUSTAL W suite of programs33 was NADPH-GOGAT small subunit serves priused to calculate the tree. The numbers correspond to ÔbootstrapÕ percentages. The length of the bar represents 0.1 substitutions per site. GOGAT protein sequence information was marily to couple the oxidation of NADPH obtained either from the GenBank protein database or from conceptual translations of to the reductive transfer of the amido group GOGAT-encoding genes found in the GenBank nucleotide database. The accession numof glutamine to the a-keto position of 2bers used were: Maize-Fd, M59190; Arabidopsis-Fd, Y09667; Porphyra-Fd (P. purpurea), oxoglutarate. U38804; Antithamnion-Fd, Z21705; Plectonema-Fd (P. boryanum), 085735; The consensus view of GOGAT strucSynechocystis-GltS, X92480; Azospirillum-NADP (A. brasilense), L04300; Escherichiature and function has recently been chalNADP (E. coli), AE000400; Pseudomonas-NADP (P. aeruginosae), U81261; lenged. Two GOGAT genes, gltB and gltS, Mycobacterium-NAD (M. tuberculosis), Z83864; Plectonema-NAD (P. boryanum), have been isolated and characterized from D85230; Synechocystis-GltB, X80485; Alfalfa-NAD, L01660; Rice-NAD, AB0011916; a Synechocystis sp.19. Both of these genes Saccharomyces-NAD (S. cerevisiae), X89221; and Caenorhabditis-NAD (C. elegans), are similar to the large subunit gene of nonZ49868 and Z49889. photosynthetic eubacteria, but one is much more similar to plant NADH-GOGAT than it is to any other Fd-GOGAT protein. This indicates that cyanobacteria might, like plants, have both Fd- and result is reminiscent of evidence obtained with E. coli NADPHNAD(P)H-GOGAT enzymes. However, significant Synecho- GOGAT showing that the gltA-encoded subunit alone can catacystis Fd-GOGAT activity was retained in mutants that contained lyze the ammonia-dependent synthesis of glutamate21. It is not insertionally mutagenized gltB and gltS genes. Additionally, known whether Pyrococcus contains a gltB-like gene. Navarro et al.19 and other researchers have been unable to detect In contrast to the findings of Jongsareejit et al.20, examination any NAD(P)H-GOGAT activity in this organism. Thus, based on of the recently sequenced Methanococcus jannaschii genome both biochemical and mutational analyses, it appears that the two revealed that this archaebacterium may not contain a gene encodSynechocystis genes encode Fd-GOGAT. However, recent analy- ing a gltA subunit. Although several genes are similar to gltA, all sis of the complete Synechocystis genomic sequence suggests that have been assigned other functions, and none is nearly as similar this organism contains a gene similar to gltA, which encodes the to E. coli gltA as E. coli gltA is to Pyrococcus gltA. Furthermore, small subunit of all eubacterial NAD(P)H-GOGAT proteins. the structure of the protein encoded by the M. jannaschii gltB-like Another test of the consensus view of GOGAT structure and gene is unusual. Comparisons of eubacterial and eukaryotic function comes from comparisons of eubacterial and eukaryotic GOGAT large subunits show that their deduced amino acid GOGAT sequences with those of archaebacteria. All examined sequences are similar throughout most of the protein, with genereubacteria and eukaryotes contain genes encoding NAD(P)H- ally more than 40% amino acid identity. This relationship does GOGAT proteins similar to E. coli gltA and gltB gene products. A not hold true when the product of the M. jannaschii gltB-like gene recent report20 describes the isolation and characterization of a is examined. Compared with the corresponding eubacterial and GOGAT gene from the archaebacterium Pyrococcus sp. KOD1. eukaryotic proteins, this protein is very small. Additionally, comThis gene encodes a protein that is very similar to the eubacterial parison of this 510 amino acid (55 kDa) protein with other gltA-encoded GOGAT small subunits. However, unlike the gltA GOGAT proteins shows that it is composed of two distinct regions gene in eubacteria, it is not linked to the gltB gene. Furthermore, that are similar to the eubacterial GltB subunit (Fig. 4). The region when the Pyrococcus gltA gene was expressed in E. coli, the puri- from approximately amino acids 90 to 160 of the archaebacterium fied product was capable of catalyzing both glutamine- and GltB-like protein is similar to a region from about 500 to 580 of the ammonia-dependent, NADPH-coupled glutamate synthesis. This eukaryotic GOGATs, and contains no known functional properties. February 1998, Vol. 3, No. 2 53 trends in plant science reviews photorespiratory carbon and nitrogen cycles. This pioneering work confirmed the existence of the photorespiratory cycle; established the importance of Fd-GOGAT in photorespiratory NH4+ reassimilation; and was instrumental in establishing Arabidopsis as a model plant system. Subsequent analysis of one of these FdGOGAT mutants indicated that it contains undetectable Fd-GOGAT protein in either leaf or root tissue, although it does retain a low level of activity and transcript24. This apparent conflict between genetic and molecular data is at least partly resolved by the recent finding that Arabidopsis has a second Fd-GOGAT gene8. The GLU2 gene9 is Fig. 4. Regions of sequence similarity among eukaryotic, eubacterial and archaebacterial expressed predominantly in roots, and has glutamate synthase (GOGAT) proteins. The black boxes represent sequences that are not very low levels of expression in leaves; found in the mature protein and that have little sequence similarity; open boxes show GLU1 is expressed predominantly in regions of sequence similarity among the alfalfa, Arabidopsis and E. coli GOGAT proteins; leaves. Presumably, GLU1 expression, but boxes filled with horizontal or diagonal lines represent locations of similarities found among all the proteins; lightly shaded boxes are regions that have little or no sequence siminot GLU2 expression, was affected in the larity with other proteins; and the box filled with vertical lines represents a region that is photorespiratory mutants. extremely hydrophilic and separates GltB- and GltA-like domains of eukaryotic NADHUsing a similar screening strategy, FdGOGAT. GOGAT mutants have been isolated from barley and pea25. Fd-GOGAT activity is virtually undetectable in the leaves of the The C-terminal 350 amino acids of the archaebacterium GOGAT barley mutants. However, levels of NADH-GOGAT are present at are similar to amino acids 880 to 1240 of eukaryotic GOGATs. This wild-type levels, indicating that no compensatory mechanisms region binds flavin mononucleotide and contains the 3Fe/4S center. exist and confirming the distinct roles proposed for the two Further research is needed to elucidate the structure and function of GOGAT isoforms. Immunoblot analysis of four barley FdGOGAT in these ÔprimitiveÕ organisms. GOGAT mutant lines indicated that three had no detectable crossreacting Fd-GOGAT protein and that the fourth had significantly Post-translational processing and intracellular localization of reduced levels of this protein26. Photosynthetic CO2 assimilation glutamate synthase in the barley Fd-GOGAT mutants is reduced on transfer from Fd-GOGAT proteins from Arabidopsis and maize contain a pre- elevated CO2 to air. This effect on photosynthesis is unlikely to be sequence with many of the characteristics of plastid transit pep- caused solely by the accumulation of NH4+ and may result from the tides, including a net positive charge and high proportion of unusual and complex interaction of carbon and nitrogen metabthreonine and/or serine residues. Consistent with the projected olism that occurs in these mutants as a result of photorespiration27. plastid localization of the plant enzyme is the plastid localization Genetic engineering techniques have recently been used to genof its cofactor, ferredoxin, and the plastid location of the Fd- erate transgenic tobacco plants that express an Fd-GOGAT cDNA GOGAT genes in red algae. Similarly, both rice and alfalfa4 fragment in the antisense orientation under the control of the NADH-GOGATs contain presequences that are thought to be CaMV-35S promoter. The five transgenic tobacco lines recovered involved in plastid targeting. Interestingly, presequences are were found to contain between 40 and 100% of wild-type levels of found in all characterized eukaryotic GOGAT proteins, including Fd-GOGAT activity and protein. Under ambient growth conditions, yeasts22 and nematodes, and all eubacterial GltB and cyanobac- severe symptoms of ammonia toxicity were seen in the two lines terial Fd-GOGAT proteins. The yeast and nematode presequences that contain less than 60% of wild-type Fd-GOGAT activity28. do not have the appearance of mitochondrial transit peptides22, In contrast to the multiplicity of mutants for Fd-GOGAT, there and thus their function is unknown. The N-terminus of all charac- are no plant mutants for NADH-GOGAT activity. The phenotype terized GltB-like proteins is a cysteine, which is thought to be a of such mutants is difficult to predict, but one might expect a subcritical component of the glutamine amidotransferase activity of stantial reduction in growth and nitrogen content, particularly in the enzyme16. It is possible that one of the functions of the pre- nodulated legumes and in those plant species that reduce NO32 in sequence is to ensure the proper proteolytic processing needed to their roots. generate this N-terminal cysteine and activate the enzyme. Regulation of GOGAT in the plant Mutants of glutamate synthase Understanding of plant nitrogen metabolism has been greatly aided by the isolation and study of mutants defective in their ability to catalyze defined biochemical reactions. Arabidopsis mutants that contained only 1Ð2% of wild-type Fd-GOGAT activity in leaves were isolated in 198023. These mutants exhibited severe stress symptoms in normal air, but grew normally when placed in an atmosphere that minimized photorespiration [0.7% (v/v) CO2]. The Fd-GOGAT mutants were part of a wide range of mutant lines that were deficient in key enzymes of the 54 February 1998, Vol. 3, No. 2 Light and a variety of metabolites have been shown to exert major regulatory controls over many metabolic pathways. It is well established that light, mediated via phytochrome, has a positive effect on the expression of the chloroplast-localized isoform of glutamine synthetase29. Recent evidence indicates that light also exerts a positive regulatory effect on the expression of FdGOGAT (GLU1) (Ref. 24). GLU2 expression is also induced by light, although the induction of this gene by sucrose in the dark indicates that the light-induced expression may in part be caused by an increase in the concentration of carbon metabolites9. A trends in plant science reviews comparison, cytosolic glutamine synthetase transcript abundance in ineffective nodules is only slightly reduced (15%) as compared to effective nodules, and aspartate aminotransferase-1 is expressed at high levels in roots and both effective and ineffective nodules. Thus, unlike other key plant genes encoding proteins involved in nitrogen assimilation, increased levels of NADHGOGAT expression were associated only with effective nodules31. These experiments show that active nitrogen fixation, and perhaps NH4+ itself or a downstream product of its metabolism, is required for maximum NADH-GOGAT gene expression. Although substantial progress has been made with the molecular genetics of primary acquisition and assimilation of nitrogen, elucidating which genes are most crucial to enhancing nitrogenuse efficiency remains an important goal. A previously poorly studied area that may assist with this effort is the signal-transduction pathway involved in the activation of the genes involved in primary nitrogen assimilation, and the associated generation of carbon skeletons. c vFig. 5. An example of the analysis of glutamate synthetase (GOGAT), glutamine synthetase (GS) and aspartate aminotransferase transcripts (AAT-1) in alfalfa roots and root nodules induced by effective and ineffective Rhizobium meliloti strains. RNA was isolated from the roots and root nodules collected 12 d after the plants were inoculated with either effective wild-type R. meliloti strain 102F51 or with ineffective strain 7154 (Nif2). A northern blot was probed for the presence of transcripts encoding GOGAT, GS and AAT-1. Following hybridization, the gel was subjected to radioanalytic analysis to provide a quantitative measure of the hybridization signal for each probe. The 100% expression values from 12 d old, wild-type nodules were 1064 cpm for GOGAT; 1988 cpm for glutamine synthetase; and 680 cpm for aspartate aminotransferase-1. recently proposed model suggests that the regulation of nitrogenassimilatory enzymes in Arabidopsis is related to light- and darkmediated stimuli interconnected to levels of organic carbon and nitrogen8,9. In another recent study, tobacco transformants with very low nitrate reductase activity were used to investigate possible coordinated regulatory signals that influence carbon and nitrogen metabolism30. Nitrate was shown to act as a signal, resulting in widespread changes in the expression of key genes in the pathways of nitrogen and carbon metabolism, including FdGOGAT. The induction of organic acid metabolism and repression in starch metabolism by nitrate could increase the availability of carbon skeletons for nitrogen assimilation30. Despite the results from many biochemical studies, and the availability of cDNA clones and antibodies to all of the enzymes involved in plant nitrogen assimilation, the question as to which enzymes are limiting and thus restricting the effectiveness of efforts to enhance nitrogen-use efficiency remains unresolved. However, studies of plant gene expression in developing alfalfa nodules suggest that NADH-GOGAT is uniquely regulated as compared to the other genes involved in nitrogen and carbon metabolism4,11. Although cytosolic glutamine synthetase, aspartate aminotransferase, phosphoenolpyruvate carboxylase and malate dehydrogenase transcripts readily accumulate in ineffective (non-N2-fixing) nodules, NADH-GOGAT transcript accumulation remains low at root background levels. It is clear that NADH-GOGAT expression is strikingly different to that of either cytosolic glutamine synthetase or aspartate aminotransferase-1 (Refs 4 and 11) (Fig. 5). Although maximum expression of NADH-GOGAT occurred in effective nodules, that in ineffective nodules and roots was only 12Ð20% of the maximum. By Acknowledgements We wish to thank many colleagues for providing reprints and communicating recent results. The work was supported by National Science Foundation Grant No. IBN-9206890. This paper is a joint contribution from the Plant Sciences Research Unit, United States Dept of Agriculture, Agricultural Research Service, and the Minnesota Agriculture Experiment Station (Paper No. 971130039, Scientific Journal Series). References 01 Vance, C.P. (1997) The molecular biology of N metabolism, in Plant Metabolism (Dennis, D.T. et al., eds), pp. 449Ð477, Longman 02 Stewart, G.R., Mann, A.F. and Fentem, P.A. (1980) Enzymes of glutamate formation: glutamate dehydrogenase, glutamine synthetase, glutamate synthase, in The Biochemistry of Plants (Vol. 5) (Miflin, B.J., ed.), pp. 271Ð327, Academic Press 03 Lea, P.J., Robinson, S.A. and Stewart, G.R. (1990) The enzymology and metabolism of glutamine, glutamate and asparagine, in The Biochemistry of Plants (Vol. 16) (Miflin, B.J. and Lea, P.J. eds), pp. 121Ð159, Academic Press 04 Gregerson, R.G. et al. (1993) Molecular characterization of NADH-dependent glutamate synthase from alfalfa nodules, Plant Cell 5, 215Ð226 05 Sakakibara, H. et al. (1991) Molecular cloning and characterization of complementary DNA encoding ferredoxin dependent glutamate synthase in maize leaf, J. Biol. Chem. 266, 2028Ð2035 06 Lea, P.J. et al. (1990) Enzymes of ammonia assimilation, in Methods in Plant Biochemistry (Lea, P.J., ed.), pp. 257Ð276, Academic Press 07 Redinbaugh, M.G. and Campbell, W.H. (1993) Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate, Plant Physiol. 101, 1249Ð1255 08 Lam, H-M. et al. (1996) The molecular-genetics of nitrogen assimilation into amino acids in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 569Ð593 09 Oliveira, I.C. et al. (1997) Molecular-genetic dissection of ammonium assimilation in Arabidopsis thaliana, Plant Physiol. Biochem. 35, 185Ð198 10 Yamaya, T. et al. (1992) Tissue distribution of glutamate synthase and glutamine synthase in rice leaves, Plant Physiol. 100, 427Ð432 11 Vance, C.P. et al. (1995) Alfalfa NADH-dependent glutamate synthase: structure of gene and importance in symbiotic N2 fixation, Plant J. 8, 345Ð358 12 Chen, F-L. and Cullimore, J.V. (1988) Two isozymes of NADH-dependent glutamate synthase in root nodules of Phaseolus vulgaris L., Plant Physiol. 88, 1411Ð1417 13 Hayakawa, T. et al. (1993) Changes in the content of two glutamate synthase proteins in spikelets of rice (Oryza sativa) plants during ripening, Plant Physiol. 101, 1257Ð1262 February 1998, Vol. 3, No. 2 55 trends in plant science reviews 14 Valentin, K., Kostrzewa, M. and Zetsche, K. (1993) Glutamate synthase is plastid-encoded in a red alga: implications for the evolution of glutamate synthase, Plant Mol. Biol. 23, 77Ð85 15 Gantt, J.S. et al. (1991) Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron, EMBO J. 10, 3073Ð3078 16 Curti, B. et al. (1996) Glutamate synthase: a complex ironÐsulphur flavoprotein, Biochem. Soc. Trans. 24, 95Ð99 17 Vanoni, M.A. et al. (1992) Characterization of the flavins and the ironÐsulfur centers of glutamate synthase from Azospirillum brasilense by adsorption, circular dichroism, and electron paramagnetic resonance spectroscopies, Biochemistry 31, 4613Ð4623 18 Knaff, D.B. et al. (1991) Spectroscopic evidence for a [3FeÐ4S] cluster in spinach glutamate synthase, J. Biol. Chem. 266, 15080Ð15084 19 Navarro, F. et al. (1995) Existence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis sp. PCC 6803. Isolation and insertional inactivation of gltB and gltS genes, Plant Mol. Biol. 27, 753Ð767 20 Jongsareetjit, B. et al. (1997) Gene cloning, sequence and enzymatic properties of glutamate synthase from the hyperthermophilic arcgaeon Pyrococcus sp. KOD1, Mol. Gen. Genet. 254, 635Ð642 21 Maentsaelae, P. and Zalkin, H. (1976) Active subunits of Escherichia coli glutamate synthase, J. Bacteriol. 126, 539Ð541 22 Filetici, P. (1996) Sequence of the GLT1 gene from Saccharomyces cervisiae reveals the domain structure of yeast glutamate synthase, Yeast 12, 1359Ð1366 23 Somerville, C.R. and Ogren, W.L. (1980) Inhibition of photosynthesis in Arabidopsis mutants lacking leaf glutamate synthase activity, Nature 286, 257Ð259 24 Suzuki, A. and Rothstein, S. (1997) Structure and regulation of ferredoxindependent glutamate synthase from Arabidopsis thaliana, Eur. J. Biochem. 243, 708Ð718 25 Lea, P.J. and Forde, B.G. (1994) The use of mutants and transgenic plants to study amino acid metabolism, Plant Cell Environ. 17, 541Ð556 26 Avila, C. et al. (1993) Cloning and sequence analysis of a cDNA for barley ferredoxin-dependent glutamate synthase and molecular analysis of photorespiratory mutants deficient in the enzyme, Planta 189, 475Ð483 27 Hausler, R.E. et al. (1994) Control of photosynthesis in barley leaves with reduced activities of glutamine synthetase or glutamate synthase, Planta 194, 406Ð417 28 Hirel, B. et al. (1997) Manipulating the pathway of ammonia assimilation in transgenic non-legumes and legumes, J. Plant Nutr. Soil Sci. 160, 283Ð290 29 Peterman, T.K. and Goodman, H.M. (1991) The glutamine synthetase gene family of Arabidopsis thaliana: light-regulation and differential expression in leaves, roots and seeds, Mol. Gen. Genet. 230, 145Ð154 30 Scheible, W-R. et al. (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco, Plant Cell 9, 783Ð798 31 Vance, C.P. et al. (1994) Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzymes involved, Plant Sci. 101, 51Ð64 32 Saitou, N. and Nei, M. (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees, Mol. Biol. Evol. 4, 406Ð425 33 Higgins, D.G. and Sharp, P.M. (1988) CLUSTAL: a package for performing multiple sequence alignments on a microcomputer, Gene 73, 237Ð244 Stephen J. Temple is at the Dept of Agronomy and Plant Genetics, University of Minnesota, St Paul, MN 55108, USA; Carroll P. Vance is at the US Dept of Agriculture, Agricultural Research Service, Plant Science Research Unit and the Dept of Agronomy and Plant Genetics, University of Minnesota, St Paul, MN 55108, USA; and J. Stephen Gantt* is at the Dept of Plant Biology, University of Minnesota, St. Paul, MN 55108, USA. *Author for correspondence (tel +1 612 625 4763; fax +1 612 625 1738; e-mail [email protected]). The protein translocation apparatus of chloroplast envelopes Lisa Heins, Ian Collinson and Jürgen Soll The evolution of the chloroplast from a photosynthetic prokaryote has resulted in the displacement of most of the prokaryote genes to the nucleus of the host eukaryote. Accordingly, the new organism has evolved targeting and translocation mechanisms on the organellar membranes for nuclear-encoded proteins. In plastids, the protein-import machinery is distinct from that of other organelles, in both composition and mechanism. Recently, proteins homologous to several subunits of the chloroplast import machinery were identified in the cyanobacterium Synechocystis PCC6803. It appears that parts of the protein-import machinery of chloroplasts are derived from ancient transport systems in cyanobacteria. These observations open up new avenues for elucidating the origin of the chloroplast membranes and functional properties of the protein-import machinery. I ndependent endosymbiotic uptake of ancient prokaryotes into an early eukaryotic host is thought to have produced two types of organelles, chloroplasts and mitochondria, that are each surrounded by a double-membrane system. Fundamental differences in structure and function reflect the different origins of the two organelles. A distinguishing feature of chloroplasts is their many subcompartments, which include the outer- and inner-envelope 56 February 1998, Vol. 3, No. 2 membranes, the thylakoid-membrane network, the interenvelope space, the stroma and the thylakoid lumen. Newly evolved proteins, employed in refining and/or regulating chloroplast function, are dependent on a specific import mechanism Ð as are those proteins encoded by genes that have become displaced, during evolution, to the nucleus. The major components that catalyse this protein translocation pathway Ð which starts in the cytosol, and Copyright © 1998 Elsevier Science Ltd. All rights reserved. 1360 - 1385/98/$19.00 PII: S1360-1385(97)01161-8