* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases
Gene therapy of the human retina wikipedia , lookup
Molecular cloning wikipedia , lookup
Transcription factor wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
DNA vaccination wikipedia , lookup
Gene nomenclature wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Epigenetics of human development wikipedia , lookup
DNA polymerase wikipedia , lookup
Gene desert wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Gene therapy wikipedia , lookup
Gene expression programming wikipedia , lookup
Non-coding DNA wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Epigenomics wikipedia , lookup
Gene expression profiling wikipedia , lookup
Point mutation wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Genome editing wikipedia , lookup
Genetic engineering wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Microevolution wikipedia , lookup
History of genetic engineering wikipedia , lookup
Designer baby wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Helitron (biology) wikipedia , lookup
Primary transcript wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
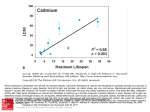
From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Type I Bovine Collagen In Vitro ModelLetters indicate statistical significance from HS27 at corresponding time point (P < .05 e and P < .001d); plus signs, statistical significance from day 2 of same cell type (P < .05c, P < .01a, and P < .001b). A, Schematic of type I bovine collagen in vitro model. Polyethylene glycol diacrylate (PEGDA) was mixed with type I collagen fibrils before encapsulation of fibroblasts (HS27 and keloid fibroblasts) through UV photopolymerization. B, Reverse transcription–polymerase chain reaction demonstrated that keloid fibroblasts had increased type I collagen gene expression compared with normal fibroblasts. C, DNA Date of download: 4/29/2017 quantification normalized to dry weight indicated decreasing trend over time. D, Glycosaminoglycan (GAG) content by both fibroblast types normalized to DNA demonstrated increased GAG production from keloid fibroblasts. E, Total collagen content by From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Reverse Transcription–Polymerase Chain Reaction of MMP Gene Expressions From Normal and Keloid Fibroblasts in the PEGCol1 ModelNo obvious differences in trends were observed between HS27 and keloid fibroblasts. β-Actin was the housekeeping gene. Date of download: 4/29/2017 From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Matrigel In Vitro ModelLetters indicate statistical significance from HS27 at corresponding time point (P < .05 c, P < .01e, and P < .001b); plus signs, statistical significance from day 1 of same cell type (P < .05a, P < .01d, and P < .001f). A, Schematic of 3dimensional Matrigel constructs. B, Reverse transcription–polymerase chain reaction of type I collagen from both HS27 and keloid fibroblasts after culture in Matrigel demonstrates the expected higher gene expression from keloid fibroblasts. C, DNA content decreased in both fibroblast types as time increased. D, Glycosaminoglycan (GAG) content was more significantly produced from Date download: than 4/29/2017 keloidoffibroblasts HS27, resulting in a time-dependent increase. E, Total collagen content normalized to DNA also demonstrated more extracellular matrix production from keloid fibroblasts than HS27. From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Reverse Transcription–Polymerase Chain Reaction of MMP Gene Expressions From Normal and Keloid Fibroblasts After In Vitro 3Dimensional Culture in MatrigelSignificant differences were observed in MMP9 and MMP13 gene expressions between HS27 and keloid fibroblasts. β-Actin was the housekeeping gene. Date of download: 4/29/2017 From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Effect of Decorin on Keloid Fibroblasts in the Matrigel ModelLetters indicate statistical significance from HS27 at corresponding time point (P < .01b); plus signs, statistical significance from day 7 of same cell type (P < .001a). A, DNA quantification normalized to respective dry weights indicated decrease in both conditions as time increased. B, Glycosaminoglycan (GAG) content normalized to DNA demonstrated that the presence of decorin prevented an increase in the matrix component production. C, Total collagen content normalized to DNA demonstrated a similar trend as the GAG data, although not statistically significant. D, Reverse Date of download: 4/29/2017 transcription–polymerase chain reaction of type I collagen and MMP genes that were affected by the presence of decorin administered to keloid fibroblasts. Significant downregulations in type I collagen, MMP1, and MMP13 were observed at day 21, From: Tissue Engineering for In Vitro Analysis of Matrix Metalloproteinases in the Pathogenesis of Keloid Lesions JAMA Facial Plast Surg. 2013;15(6):448-456. doi:10.1001/jamafacial.2013.1211 Figure Legend: Site-Specific Gene ExpressionsA, Different sites from which keloid fibroblasts were isolated from the shoulder lesion for comparison of MMP gene expressions among the different sites: i, superficial side; ii, superficial center; iii, deep center; and iv, keratinocytes. Keratinocytes were also isolated from the shoulder lesion. B, Reverse transcription–polymerase chain reaction of MMP gene expressions from primary fibroblasts isolated from different sites of a keloid lesion and compared with the corresponding keratinocytes that lined the epidermis of the lesion, a mixed fibroblast population from the ear, and normal fibroblasts isolated from Date of download: 4/29/2017 from regions closest to the margins of the original wound and the excision site (ie, superficial side and an eyebrow lift. Fibroblasts deep center) had higher expressions of MMP1, MMP2, MMP3, and MMP9 compared with those farthest away from the wound