* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cellular Respiration
Gaseous signaling molecules wikipedia , lookup
Signal transduction wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Phosphorylation wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
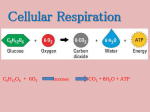
Cellular Respiration The metabolic processes within cells that makes ATP using energy from organic molecules such as glucose. The need for respiration Adenosine triphosphate (ATP) the short-term energy store of all cells easily transported and is therefore the universal energy carrier formed from the nucleotide adenosine monophosphate by the addition of two further phosphate molecules a metabolically active cell may require up to 2 million ATP molecules every second!!!!!!!! Adenosine triphosphate (ATP) Adenosine triphosphate (ATP) Adenosine triphosphate (ATP) Cellular Respiration Why can cells obtain energy from oxidising molecules such as glucose? The carbon and hydrogen atoms in cells, for example in glucose molecules, are not in their most stable form The most energetically stable form of carbon is carbon dioxide and the most energetically stable form of hydrogen is water. A cell can therefore obtain energy from sugar molecules (or amino acids or fatty acids) by allowing the carbon and hydrogen atoms in these molecules to combine with oxygen to form carbon dioxide and water. This oxidation occurs through a series of steps called aerobic respiration Overall: C6H12O6 + 6O2 6CO2 + 6H2O How is this different from burning glucose? Burning glucose: C6H12O6 CO2 + H2O one step oxidation Respiration: C6H12O6 CO2 + H2O multi-step catabolism Cellular Respiration This can be divided into 4 main stages: Glycolysis Link reaction Krebs cycle Electron transport chain Just to confuse you!!! The stages of respiration have other names Glycolysis - the Embden-Meyerhof pathway Krebs cycle - the citric acid cycle - the tricarboxylic acid (TCA) cycle Electron transport chain - the hydrogen carrier system - the cytochrome system Where it all happens: Glycolysis Link reaction Krebs cycle Glycolysis – in the cytoplasm Electron transport chain Link reaction and Krebs cycle in the matrix of the mitochondria E.T. chain on the inner membrane of the mitochondria Summary of events Substrates glucose Stages glycolysis pyruvate link reaction Products ATP carbon dioxide acetyl CoA Krebs cycle carbon dioxide & ATP H Atoms (H+ & e-) oxygen electron transport chain water & ATP Key terms: ATP adenosine triphosphate NAD+ / NADH oxidised NAD/ reduced NAD a coenzyme FAD/ FADH2 oxidised FAD/ reduced FAD a coenzyme substrate level phosphorylation where a substrate molecule ( X-p ) donates its P to ADP making ATP oxidative phosphorylation the production of ATP as a result of energy released during an oxidation reaction dehydrogenation the removal of hydrogen in a reaction decarboxylation the removal of CO2 in a reaction coenzyme a small molecule that is required to bind to an enzyme to ensure its correct functioning; e.g. NAD & FAD Oxidation and reduction understanding these two terms is central to understanding respiration Oxidation is the loss of electrons Reduction is the gain of electrons OILRIG Oxidation and reduction NAD [and FAD] act as ‘hydrogen carriers’ F214 Glycolysis 1 glyco - ‘sugar’ ; lyso - ‘breakdown’ the breakdown of a hexose sugar into two molecules of the three-carbon compound pyruvate (pyruvic acid) occurs in all cells gives a net gain of 2 ATP molecules in anaerobic respiration it is the only stage of respiration Glycolysis 2 look at the summary of glycolysis on the worksheet do not try to memorise this sequence of reactions concentrate on understanding what happens in this stage of respiration and identifying the important points One molecule of glucose(with 6 C atoms) is broken down into two molecules of pyruvate (with 3 C atoms each) Two molecules of ATP are used in the very first steps but later four molecules are synthesised by substrate level phosphorylation ( a net gain of two ATP molecules) hydrogen atoms are removed from glucose molecules when two molecules of NAD+ are reduced to NADH Summary of glycolysis Substrate-level phosphorylation Enzyme P P adenosine ADP substrate product P P P P ATP adenosine Energy changes in glycolysis The stages of glycolysis that involve energy changes are shown below: hexose 1,6-biphosphate glucose 6-phosphate glucose 2 x glycerate 3-phosphate 2 x pyruvate Reaction time The story so far; 1 ……………………. One molecule of glucose (6c) Glycolysis 2 molecules of reduced NAD (NADH) 2 molecules of ATP (net gain) 2 molecules of pyruvate (3C) cytoplasm Link reaction matrix of mitochondrion 2 molecules of reduced NAD (NADH) 2 molecules of acetyl CoA (2C) 2 molecules of CO2 The Link Reaction Link reaction Link reaction- formation of Acetyl CoA This occurs in the fluid filled centre (matrix) of mitochondria The two pyruvate molecules produced by glycolysis enter the mitochondrial matrix from the cytoplasm Here each pyruvate is converted to acetyl coenzyme A (usually written as acetyl CoA) In this reaction carbon dioxide is given off and each pyruvate loses a pair of hydrogen atoms which results in reduced NAD (NADH) Acetyl CoA is a complex molecule incorporating a coenzyme derived from pantothenic acid (vitamin B5) Link reaction pyruvate + coenzyme A acetyl coenzyme A + CO2 3C 2C Coenzyme A [CoA] 1C Link reaction- formation of Acetyl CoA The story so far; 2 ……………………. One molecule of glucose (6c) Glycolysis 2 molecules of reduced NAD (NADH) 2 molecules of ATP (net gain) 2 molecules of pyruvate (3C) cytoplasm Link reaction matrix of mitochondrion 2 molecules of CO2 2 molecules of reduced NAD (NADH) 2 molecules of acetyl CoA (2C) Krebs cycle 1 important for three main reasons: enables breakdown of macromolecules with the release of CO2 provides reducing power for electron transport system - produces pairs of hydrogen atoms which are ultimately the source of metabolic energy for the cell acts as an interconversion centre - is a valuable source of intermediate compounds used in making e.g. fatty acids, amino acids Krebs cycle 2 This series of reactions also occurs in the matrix of mitochondria Each molecule of acetyl CoA combines with a 4-C compound (oxalocacetate) to form a 6-C compound (citrate) This citrate then undergoes four dehydrogenations the removal of hydrogen and then two decarboxylations – the removal of carbon dioxide Krebs cycle - detail Krebs cycle Krebs cycle as a result of these reactions: oxaloacetate is regenerated and can start the cycle off again three molecules of NAD are reduced to NADH one molecule of FAD is reduced to FADH2 two molecules of CO2 are produced one molecule of ATP is made by substrate level phosphorylation Q How many times will the cycle ‘turn’ during the catabolism of one molecule of glucose? 2 Krebs cycle The overall reaction accomplished by two ‘turns’ of the Krebs cycle is: 2 acetyl CoA + 6NAD + 2FAD + 2ADP + 2Pi 4CO2 + 6NADH + 2FADH2 + 2ATP The story so far; 3……………………. One molecule of glucose (6c) Glycolysis 2 molecules ATP (net) 2 molecules of NADH 2 x pyruvate (3C) cytoplasm Link reaction matrix of mitochondrion 2 molecules of CO2 2 molecules of NADH 2 x acetyl CoA (2C) Krebs Cycle 4 molecules of CO2 2 molecules of ATP 2 molecules of FADH2 6 molecules of NADH Electron transport chain 1 the means by which the energy, in the form of hydrogen atoms, mostly from the Krebs cycle, is converted to ATP hydrogen atoms (H+ and e- ), attached to carriers, are passed along a chain of progressively lower energy levels (the respiratory chain) the energy released is harnessed to produce ATP through the oxidation of the hydrogen atoms (oxidative phosphorylation) Electron transport chain 2 Look back at the equations representing the catabolism of a molecule of glucose so far and you will notice that a number of reduced coenzyme molecules (either NADH or FADH2) have been produced as the glucose molecule has been stripped of its electrons Q how many molecules of NADH have been produced? Q how many molecules of FADH2 have been produced? 10 NADH; 2 FADH2 Electron transport chain 3 In the electron transfer chain these reduced coenzymes are re-oxidised. The enzymes which catalyse this re-oxidation process are located on the folded inner mitochondrial membrane where they are arranged in a series. Q What is the likely advantage of this high degree of folding of the inner membrane of the mitochondria to form cristae? increased surface area for the location of the enzymes of the electron transport chain Electron transport chain 4 The overall reaction of the electron transport chain: 10NADH + 2FADH2 + 6O2 10NAD + 2FAD + 12H2O During this oxidative reaction, ATP is produced by oxidative phosphorylation Electron transport chain 5 The last cytochrome in the chain is re-oxidised by donating its electrons to oxygen thus producing water - the other waste product of respiration Voila!!!! Respiration is complete. Well, almost ……. How exactly is this ATP made? The chemiosmosis theory…….. H H+ + e- chemiosmosis chemiosmosis chemiosmosis chemiosmosis oxidative phosphorylation chemiosmosis oxidative phosphorylation chemiosmosis oxidative phosphorylation formation of water chemiosmosis How much ATP? 10 molecules of reduced NAD ; average 2.5 ATP molecules from each = 25 ATP 2 molecules of reduced FAD ; average 1.5 ATP molecules from each = 3 ATP Added to the 2ATP from glycolysis and 2ATP from Krebs cycle Total = 32ATP from one molecule of glucose How much ATP? Evidence for chemiosmosis Evidence for chemiosmosis Evidence for chemiosmosis Murder most foul A favourite poison for murderers in crime fiction is cyanide because it works so rapidly. But why is this substance so poisonous? The cyanide ion (CN-) binds irreversibly to the enzyme that catalyses the oxidation of cytochrome a (cytochrome oxidase) As a result, cyanide stops the flow of electrons essential to oxidative phosphorylation. This prevents the re-oxidation of NADH and FADH and thus the production of the majority of the cell’s ATP Without ATP muscles go into a permanent state of contraction leading to the characteristic arching of the back and death by asphyxiation since the muscles needed for breathing no longer function. http://www.indiana.edu/~oso/animations/An7.html Summary LINK REACTION Phew! I need a lie down