* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biotransformation Xenobiotic metabolism
Multi-state modeling of biomolecules wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Metabolomics wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Citric acid cycle wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Glutathione wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
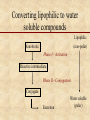
Biotransformation Xenobiotic metabolism “Essentials of Toxicology” by Klaassen Curtis D. and Watkins John B Chapter 6 Biotransformation • Water soluble xenobiotics are easier to eliminate ( t1/2) – Urine, feces but not exhalation – If within barrier, no out • Multiple enzymes (families) – – – – – Constitutively expressed Inducible Broad specificity Polymorphic (allelic variants) Stereo-isomer specificity: 6-OH in hormones: CYP2A1 6-OH CYP3A 6-OH Biotransformation Potentially toxic xenobiotic Detoxification Inactive metabolite Relatively harmless Metabolic activation Reactive intermediate Converting lipophilic to water soluble compounds Lipophilic Xenobiotic (non-polar) Phase I - Activation Reactive intermediate Phase II - Conjugation Conjugate Excretion Water soluble (polar) Phase I • introduction of functional group • hydrophilicity increases slightly • may inactivate or activate original compound • major player is CYP or mixed function oxygenase (MFO) system in conjunction with NAD(P)H • location of reactions is smooth endoplasmic reticulum Phase II • conjugation with endogenous molecules (GSH, glycine, cystein, glucuronic acid) • hydrophilicity increases substantially • neutralization of active metabolic intermediates • facilitation of elimination • location of reactions is cytoplasm Phase I reactions Table 6.1 Oxidation Hydroxylation (addition of -OH group) N- and O- Dealkylation (removal of -CH side chains) Deamination (removal of -NH side chains) Epoxidation (formation of epoxides) C Oxygen addition (sulfoxidation, N-oxidation) Hydrogen removal O epoxide C Reduction Hydrogen addition (unsaturated bonds to saturated) Donor molecules include GSH, FAD, NAD(P)H Oxygen removal Hydrolysis C O Splitting of C-N-C (amide) and C-O-C (ester) bonds See also Chapter 6 of Casarett and Doull’s “Toxicology” Biotransformation • Activation of xenobiotics is a key element (e.g. benzene, vinyl chloride) – Reactive intermediates include epoxides and free radical species (unpaired electrons) that are short-lived and hence highly reactive – Protection is provided by • endogenous antioxidant substances, e.g. GSH • vitamins C and E • antioxidant enzymes, SOD, GPX, CAT in coupled reactions – Antioxidant molecules are oxidized in the process but have the capacity to regenerate the reduced form from the oxidized - NAD(P)H is a key player See also p. 40-44 of Casarett and Doull’s “Toxicology” Cytochrome P450 (CYP) Mixed Function Oxidases (MFO) • • • • • Located in many tissues but highly in liver ER Human: 16 gene families CYP 1,2,3 perform drug metabolism >48 genes sequenced Key forms: CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 • Highly inducible – Alcohol – Dioxin/PCBs – Barbiturates CYP2E1 CYP1A CYP2B • CYP genes have multiple alleles (2D6 has 53, and 2E1 has 13) The CYP-450 reaction cycle A G (B) C F E D Oxidation of vinyl chloride to an epoxide Metabolic enzymes 1. Microsomal: 1. 2. 2. Non-microsomal 1. 2. 3. 3. CYP450 monooxygenases Flavin monooxygenase Alcohol dehydrogenase Aldehyde dehydrogenase Monoamine and diamine oxidases Both 1. 2. 3. Esterases and Amidases Prostaglandin synthase Peroxidases Cooxidation of acetaminophen by prostaglandin endoperoxide synthetase Compare to fig. 6-2 Hydrolysis of esters and amides Hydrolysis of organophosphates Hydrolysis of epoxides Stereoselective hydroxylation Metabolism of benzo(a)pyrene to 9,10 epoxide: Potent mutagen that binds DNA Azo- and nitro- reduction Intestinal flora as part of biotransformation Ready for elimination Flora action Oxidation reactions Benzene trasformation to leukemia-causing metabolite Flavin mono-oxygenases (FMO) catalyzed reactions Nitrogen compounds Phase II reactions • • • • • Glycoside conjugation - glucuronidation Sulfate - sulfation Glutathione (GSH) Methylation Acylation – Acetylation – Amino acid conjugation – Deacetylation • Phosphate conjugation Glucuronidation of phenol Sulfation of phenol and toluene GSH conjugation of acetaminophen Glutathione -glutamyl-cysteinyl-glycine Active site of a GST: