* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
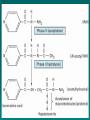
Biotransformation Siva Nageswararao Mekala Assistant Professor Dept. of clinical Pharmacology Biotransformation It is the chemical transformation of the drug within the living organism What happens to metabolized drug ? Lipid soluble and Unionized compounds Converted Water soluble or more ionized compounds reducing renal tubular reabsorption facilitating their excretion Drug metabolism does not always result in detoxification and inactivation of drugs Foreign chemicals or xenobiotics (substances foreign to the body) Environmental contaminants as well as in our diets. Plants (phytoallexins) eg, poisonous mushrooms Drugs are considered xenobiotics Most are extensively metabolized in humans. Humans exposed Environmental pollution, food additives, cosmetic products, agrochemicals, processed foods, and drugs. ( lipophilic chemicals) If there is absence of metabolism accumulate in the body, Resulting in toxicity Biotransformation reactions may have four different consequences with respect to pharmacologic activity: i. ACTIVATION: An inactive precursor is converted into pharmacologically active drug L-dopa (inactive) to Dopamine(active) prednisone(inactive) to prednisolone(active) Such inactive drugs are known as PRODRUGS Prodrugs have following advantages: Active form is more stable Better bioavailability Desired pharmacokinetics Less side effects and toxicity e.g., Enalapril – Enalaprilat Bacampicillin – Ampicillin Acyclovir- Acyclovir triphosphate ii. MAINTENANCE OF ACTIVITY: An active substance is converted into active metabolite e.g: Diazepam to Oxazepam Codeine to Morphine iii. INACTIVATION: An active drug is converted to inactive products e.g: Pentobarbital to Hydroxypentobarbital iv. ACTIVE DRUG TO HIGHLY TOXIC METABOLITE : eg: Paracetamole converts N-acetyl-p-benzoquinoneimine Sites Metabolizing enzymes are found in most tissues in the body with the highest levels located in the tissues of the gastrointestinal tract [liver, small and large intestines] For orally administered drug---- LIVER Lower gut In addition, drugs may be metabolized by gastric acid (eg, penicillin) digestive enzymes (eg, polypeptides such as insulin) enzymes in the wall of the intestine (eg, sympathomimetic catecholamines Other organs that contain significant xenobioticmetabolizing enzymes include the tissues of the nasal mucosa and lung Biotransformation reactions can be assigned to two major categories: PHASE - I (NON-SYNTHETIC REACTIONS): Convertion of the parent drug to a more polar metabolite by introducing or unmasking a functional group (–OH, –NH2, –SH). PHASE - II(SYNTHETIC REACTIONS): Enzymes form conjugate of the substrate NOTE: Drugs originally containing reactive groups NH2 , OH or COOH - can undergo direct phase 2 reactions 5/9/2017 PHASE I In this enzymes carry out Oxidation: Addition of Oxygen and/or removal of Hydrogen . It is the most important and common metabolic reaction. Reduction: Removal of oxygen or addition of hydrogen Hydrolytic reactions: Breakdown of the compound by addition of water Cyclization: This is formation of ring structure from a straight chain compound Decyclization : This is opening up of ring structure of the cyclic drug molecule. OXIDATIVE REACTIONS: Eg: Phenytoin ,Phenobarbitone ,Propranolol, pentobarbitone REDUCTION REACTIONS: Eg: Chloramphenicol , methadone , Halothane ,Warfarin HYDROLYSIS REACTION: Eg: Esters: Procaine , Succinylcholine Amides: lignocaine ,Procainamide CYCLIZATION: Proguanil DECYCLIZATION: Barbiturates ,phynytoin 5/9/2017 Classes of enzymes: i. Cytochrome P450s (P450 or CYP), ii Flavin-containing monooxygenases (FMO) iii Epoxide hydrolases - Hydrolysis of epoxides (mES, sES) 5/9/2017 CYTOCHROME P(CYP) The CYPs are a superfamily of enzymes, all of which contain a molecule of heme that is noncovalently bound to the polypeptide chain CYPs use O2, plus H+ derived from the NADPH to carry out the oxidation of substrates 5/9/2017 CYPs metabolism of dietary and xenobiotic synthesis of endogenous compounds CYPs (> 50) such as steroids, estrogens metabolism of bile acids Specificity CYP 19 Testosterone Aromatase (member of the cytochrome P450 superfamily) Estrogen(estradiol) CYP3A4 gene number 4 subfamily A family 3 www.themegallery.com CYP 3A4 In humans the 12 CYPs that are important are: CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, and 3A5 CYP1A2, 15% CYP2A6, 4% CYP2C9, 20% CYP2D6, 5% CYP2E1, 10% CYP3A4 30% PHASE II In Phase II consists of Conjugation reaction. Phase 2 "transferases“ Sulfotransferases (SULT) UDP-glucuronosyl transferases (UGT) Glutathione-Stransferases (GST) N-acetyltransferases (NAT) Methyltransferases (MT) Addition of sulfate Addition of glucuronic acid Addition of glutathione Addition of acetyl group Addition of methyl group PHASE II REACTIONS Parent drugs or their phase I metabolites undergo coupling or conjugation reactions with an endogenous substance to yield drug conjugate Conjugates are polar molecules that are readily excreted and often inactive. 5/9/2017 Type of Conjugation Endogenous Reactant Examples Glucuronidation UDP glucuronic acid (Hydroxyl or carboxyl acid group) Acetylation Acetyl-CoA (Amino or hydrazine residues) Glutathione Conjugation (GSH), morphine diazepam isoniazid, dapsone Glutathione (Quinone or epoxide reactive intermediates) Acetaminophen Type of Conjugation Endogenous Reactant Examples Glycine GlycineAcyl -CoA Conjugation (Having carboxylic acid group)-minor pathway Salicylic acid nicotinic acid Sulfation (Phenolic or steroids) Estrone methyldopa Phosphoadenosyl phosphosulfate S-Adenosyl Methylation methionine, Dopamine epinephrine, histamine (Amines and phenols) Water Conjugation Water Benzopyrene Leukotriene A4 Phenytoin (highly lipophilic) CYP (PHASE I) 4-hydroxyphenytoin (Slightly water soluble) UGT+UDP-GA (PHASE II) 4-hydroxyphenytoin (highly water soluble) beta-d-glucuronide Drug metabolizing enzymes Microsomal Located in smooth ER Catalyzes phaseI and Phase II glucuronidation INDUCIBLE BY DRUGS Non microsomal Cytoplasm and mitochondria Catalyzes all conjugation reactions except glucuronidation NON- INDUCIBLE BY DRUGS Hofmann Elimination Inactivation of a drug in the body fluids by spontaneous molecular rearrangement without the agency of enzyme (Non enzymatic degradation in the plasma) E.g., ATRACURIUM - cleavage of the N-alkyl portion in the benzylisoquinoline ENZYME INDUCTION Repeated administration of certain drugs increases the synthesis of microsomal enzymes - Chemically induce P450 enhancing the rate of its synthesis reducing its rate of degradation Induction involves microsomal enzymes in liver and other organs It increases rate of metabolism by 2-4 fold Various substrates inducing P450 are Drugs: inducing CYP3A4 are Barbiturates, carbamazepine, phenytoin, rifampin Environmental pollutants: induce CYP1A1/2 enzymesactivation of procarcinogens Eg: tobacco smoke, charcoal-broiled meat, polycyclic hydrocarbons Clinical relevance of enzyme induction a) Drug drug interactions: Oral contraceptive and rifampicin : Rifampicin induces the drug metabolizing enzyme of oral contraceptives, thus enhancing the metabolism and leading to contraceptive failure. Phenytoin accelerates metabolism of vit.D3 and lead to osteomalacia c) Therapeutic benefits: To treat neonatal jaundice - phenobarbitone can be given to infant soon after birth to induce hepatic glucuronyl transferase (Bilirubin is conjugated and excreted) - In Cushing’s syndrome (Abnormally high levels of cortisol) phenytoin may reduce the manifestations by enhancing degradation of steroids - Chronic poisoning : faster metabolism of accumulated poisonous substance - Vegetables like cabbage ,spinach etc can induce microsomal enzymes and promotes the rapid elimination of the drugs ENZYME INHIBITION Certain drug substrates inhibit cytochrome P450 enzyme activity cimetidine and ketoconazole bind tightly to the P450 heme iron reduce the metabolism of endogenous substances competitive inhibition. Factors affecting drug biotransformation AGE: Increased susceptibility to the pharmacologic or toxic activity of drugs is seen in young and very old patients compared with young adults .In neonates mainly due to diminished activity of hepatic microsomal enzymes. SEX: dependent differences in drug metabolism exist in humans for ethanol, propranolol, some benzodiazepines, estrogens, and salicylates. N-Demethylation of erythromycin F>M. Propranolol oxidation M>F. This is most probably due to hormonal differences • DIET AND ENVIRONMENTAL FACTORS Charcoal-broiled foods and cruciferous vegetables induce CYP1A enzymes Grapefruit juice inhibits the CYP3A metabolism of coadministered drug substrates Cigarette smokers metabolize some drugs more rapidly than nonsmokers because of enzyme induction •DRUG DRUG INTERACTIONS DURING METABOLISM: Enzyme-inducing drugs like sedative-hypnotics, antipsychotics, anticonvulsants, the antitubercular drug rifampin, and insecticides may increase the requirement dose of drugs like warfarin •DISEASES AND METABOLISM Disease like alcoholic hepatitis, cirrhosis impair hepatic drug metabolising enzymes E.g. half-life of diazepam in patients with liver abnormality are greatly increased, with a corresponding prolongation of their effects •GENETIC VARIATION (Pharmacogenetics) Genetic factors that affect enzyme level leads to difference in metabolism Defect Enzyme Involved N-Acetylation N-acetyl transferase Ester hydrolysis Plasma cholinesterase Drug Clinical Consequences Isoniazid Peripheral neuropathy Sch Prolonged apnea G6PD Deficiency –Hemolysis when exposed to certain drugs like Sulphonamides , Salicylates THANK YOU…! 5/9/2017