* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Dickkopf1 Is Required for Embryonic Head Induction
Preimplantation genetic diagnosis wikipedia , lookup
Microevolution wikipedia , lookup
X-inactivation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Genomic imprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Wnt signaling pathway wikipedia , lookup
Gene expression profiling wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
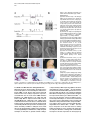
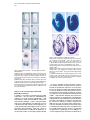
Developmental Cell, Vol. 1, 423–434, September, 2001, Copyright 2001 by Cell Press Dickkopf1 Is Required for Embryonic Head Induction and Limb Morphogenesis in the Mouse Mahua Mukhopadhyay,1,6 Svetlana Shtrom,1,6 Concepcion Rodriguez-Esteban,2,6 Lan Chen,1 Tohru Tsukui,2 Lauren Gomer,1 David W. Dorward,3 Andrei Glinka,4 Alexander Grinberg,1 Sing-Ping Huang,1 Christof Niehrs,4 Juan Carlos Izpisúa Belmonte,2,7 and Heiner Westphal1,5,7 1 Laboratory of Mammalian Genes and Development National Institute of Child Health and Human Development National Institutes of Health Bethesda, Maryland 20892 2 Gene Expression Laboratory The Salk Institute for Biological Studies La Jolla, California 92037 3 Rocky Mountain Laboratories National Institute of Allergy and Infectious Diseases National Institutes of Health Hamilton, Montana 59840 4 Division of Molecular Embryology Deutsches Krebsforschungszentrum Im Neuenheimer Feld 280 69120 Heidelberg Germany Summary Dickkopf1 (Dkk1) is a secreted protein that acts as a Wnt inhibitor and, together with BMP inhibitors, is able to induce the formation of ectopic heads in Xenopus. Here, we show that Dkk1 null mutant embryos lack head structures anterior of the midbrain. Analysis of chimeric embryos implicates the requirement of Dkk1 in anterior axial mesendoderm but not in anterior visceral endoderm for head induction. In addition, mutant embryos show duplications and fusions of limb digits. Characterization of the limb phenotype strongly suggests a role for Dkk1 both in cell proliferation and in programmed cell death. Our data provide direct genetic evidence for the requirement of secreted Wnt antagonists during embryonic patterning and implicate Dkk1 as an essential inducer during anterior specification as well as a regulator during distal limb patterning. Introduction Wnt glycoproteins are growth factors that signal through seven transmembrane receptor proteins of the Frizzled class (Cadigan and Nusse, 1997; Moon et al., 1997a; Bhanot et al., 1996). Wnt proteins are implicated in diverse developmental processes during embryonic patterning such as anteroposterior axis formation, generation of cell polarity, and specification of cell fate (reviewed by Cadigan and Nusse, 1997; Moon et al., 5 Correspondence: [email protected] These authors contributed equally to this work. 7 The laboratories of these authors contributed equally to this study. 6 1997a). Notably, both the activation and inhibition of Wnt signaling are essential for proper anteroposterior axis formation in the vertebrate embryo for specification of caudal and rostral structures, respectively (reviewed by Niehrs, 1999). A variety of secreted Wnt inhibitors has been identified that directly bind and inactivate Wnt proteins. Among them, sFRP (Leyns et al., 1997; Rattner et al., 1997; Wang et al., 1997), WIF (Hsieh et al., 1999), and Cerberus (Glinka et al., 1997; Piccolo et al., 1999) are related to the extracellular domains of Fz receptors (Moon et al., 1997b; Wodarz and Nusse, 1998). Cerberus is a multifunctional inhibitor of BMP, Nodal, and Wnt signals (Piccolo et al., 1999). Wif-1 contains domains with homology to EGF repeats (Hsieh et al., 1999). These Wnt inhibitors have been implicated in the repression of the canonical Wnt/-catenin signaling pathway. Dickkopf1 (Dkk1) is another secreted Wnt inhibitor and member of a distinct multigene family. The Dkk genes encode proteins that contain two conserved cysteine-rich domains (Glinka et al., 1998; Krupnik et al., 1999). During vertebrate embryogenesis, Dkks are differentially expressed in various neural and mesenchymal tissues (Grotewold et al., 1999; Hashimoto et al., 2000; Monaghan et al., 1999), suggesting that the proteins are involved in many inductive processes. In Xenopus, ectopic expression of Dkk1 results in the inhibition of all Wnts tested that transduce via -catenin including Wnt8, Wnt1, Wnt3a, and Wnt2b (Glinka et al., 1998; Kazanskaya et al., 2000; Krupnik et al., 1999; Wu et al., 2000). In contrast, Dkk2 cooperates with Fz receptors to activate Wnt/-catenin signaling when overexpressed in early Xenopus embryos, suggesting that mutual antagonism between Dkk1 and Dkk2 may regulate Wnt/-catenin signaling (Wu et al., 2000). Wnt inhibitors have been implicated in the regulation of various embryonic patterning processes. Dkk1 and Crescent are capable of inducing the heart Anlage in chick (Marvin et al., 2001; Schneider and Mercola, 2001). sFRP2 is involved in the patterning of avian hindbrain rhombomeres (Ellies et al., 2000). In Xenopus, Cerberus, Frzb, and Dkk1 are thought to antagonize Wnts that inhibit the Spemann organizer during embryonic head induction. That this pathway is capable of antagonizing the head organizer is suggested by the overexpression of Wnts during gastrulation, which leads to microcephaly in Xenopus (Christian and Moon, 1993; McGrew et al., 1997) and possibly in mouse (Pöpperl et al., 1997). Conversely, the overexpression of Dkk1 results in enlarged heads in Xenopus and zebrafish embryos (Glinka et al., 1998; Hashimoto et al., 2000; Shinya et al., 2000). Furthermore, in Xenopus, coexpression of Dkk1 with inhibitors of the BMP signaling pathway leads to ectopic head induction (Glinka et al., 1998). The suggestion that Dkk1 acts as a head inducer in the Xenopus organizer is consistent with its expression in the anterior endomesoderm of the Spemann organizer, which characteristically harbors head-inducing activity (Bradley et al., 1996; Schneider and Mercola, 1999; Zoltewicz and Gerhart, 1997). Hence, it has been proposed that the Developmental Cell 424 amphibian head organizer acts by simultaneous inhibition of Wnt and BMP signaling and that Dkk1 plays a key role in this process (Glinka et al., 1998; Niehrs, 1999). In contrast to Xenopus, the role of Dkk1 in mammals is unknown. In the mouse, Dkk1 is first expressed in the anterior domain of the gastrulating embryo (Glinka et al., 1998; Pearce et al., 1999; Zakin et al., 2000). In this domain, head induction is thought to be mediated by the anterior visceral endoderm (AVE), an extraembryonic tissue essential for initiating head formation in mammalian embryos (Beddington and Robertson, 1998), and the anterior mesendoderm (AME), a node-derived embryonic tissue involved in anterior specification (Camus and Tam, 1999; Shawlot et al., 1999; Tam and Steiner, 1999). Murine Dkk1 inhibits the axis-inducing ability of XWnt8 in Xenopus embryos, indicating that the mouse gene functions as a Wnt inhibitor comparable to its Xenopus homolog (Glinka et al., 1998). So far, there is no genetic evidence that Wnt inhibitors play a role in anteroposterior patterning in the mouse. For example, no axis defects have been noted in mice that lack the function of the Cerberus homolog Cer1 (Belo et al., 2000; Shawlot et al., 2000; Stanley et al., 2000). Also, there is as yet no genetic evidence implicating Wnt signaling in antagonizing head induction during gastrulation, although a requirement has been established for Wnt/-catenin signaling during early axis and node induction (Huelsken et al., 2000; Liu et al., 1999). We therefore generated mice with a Dkk1 null mutation in an effort to establish the function of this gene in the early mouse embryo. Dkk1 knockout mice have two major phenotypes: they lack anterior head structures and they display forelimb malformations. Our results reveal a requirement for the inhibition of Wnt signaling during mouse axis formation and limb morphogenesis. Results Generation of Mice with a Deletion in the Dkk1 Gene In order to investigate the requirement of Dkk1 for head induction, we generated a Dkk1 null mutation via homologous recombination in embryonic stem (ES) cells (Figures 1A and 1B). The targeting vector was constructed by replacing the entire coding sequence of the Dkk1 gene with a neomycin resistance gene flanked by 3.8 kb (5⬘) and 2.4 kb (3⬘) of homologous sequences and by the thymidine kinase (Tk) gene. The targeting vector was linearized and electroporated into the R1 line of ES cells (Nagy et al., 1993). Recombinant cells were selected in the presence of G418 (for neomycin resistance) and gancyclovir (for eliminating TK-sensitive cells). Out of 200 recombinant clones screened by Southern hybridization, seven positive clones were identified that showed sitespecific recombination. ES cells expanded from two of the seven positive clones were injected into host C57BL/ 6 blastocysts. Both cell lines gave rise to chimeras that transmitted the mutated allele to their offspring. Anterior Defects in Dkk1⫺/⫺ Mice Genotypic analysis of embryos from day 7.5 to 17.5 postcoitum showed the expected Mendelian ratio; however, all homozygous mutants die at birth. Morphological defects in Dkk1 mutants are first detectable at E8.5, when a significant reduction in rostral tissue is readily apparent in homozygous embryos. By E9.5 and later, the severity of the Dkk1 mutant phenotype is quite striking. Null mutant mice lack most head structures anterior of the otic vesicle, including eyes, olfactory placodes, frontonasal mass, and the mandibular processes (Figures 1C–1E). Truncation of forebrain and portions of midbrain is clearly visible by E10.5 (Figure 1F), and the absence of all craniofacial structures anterior to the external ear is especially pronounced late in gestation (Figures 1G and 1H). The rest of the anteroposterior body axis, as well as left-right asymmetry, appear normal by gross morphology (Figures 1F–1H) and histological analysis (data not shown), and embryos have a beating heart. Gross analysis of Dkk1⫹/⫺ mice did not reveal other phenotypes that can be attributed to the targeted disruption of the gene. Skeleton staining of Dkk1⫺/⫺ embryos reveals the absence of skull derivatives anterior of the parietal bone, including nasal, mandibular, and maxillary bones (Figures 1I–1K). The parietal and interparietal bones are reduced compared to wild-type controls. Histological analysis of E11.5 embryos, using tissue-specific molecular markers, indicates a lack of telencephalon and diencephalon (Figures 2A and 2B) as well as part of the midbrain. In E11.5 mutant mice, expression of the forebrain marker Bf1 (Tao and Lai, 1992) is absent (Figures 2I and 2J). Sonic hedgehog (shh; Echelard et al., 1993) shows the expected expression in the dorsal midline up to the rostral limit of mutant embryos (Figures 2C and 2D). Expression of En2 (Joyner and Martin, 1987) confirms that the midbrain/hindbrain boundary is still present in Dkk1 null mutant mice (Figures 2E and 2F). Otx2, known to be expressed in the midbrain (Simeone et al., 1993), is also present in mutant embryos (Figures 2G and 2H). We conclude that Dkk1 is required for the formation of head derivatives anterior of the midbrain. Forebrain Patterning Defect in Dkk1⫺/⫺ Mice To investigate forebrain induction and patterning in Dkk1 null mutants, we examined the expression of a number of diagnostic molecular markers at E7.5 and E8.5. Hesx1 is required in the anterior neuroectoderm (ANE) for mammalian forebrain development (Dattani et al., 1998) and is the earliest marker for ANE (Thomas and Beddington, 1996). In Dkk1⫺/⫺ embryos, Hesx1 expression was not detected at E7.5, the late streak stage (Figures 3A and 3B) and at the headfold stage (Figures 3G and 3H). In contrast, the expression pattern of Otx2 (Ang et al., 1994; Acampora et al., 1995) was indistinguishable from that of wild-type embryos (Figures 3C and 3D). Expression of Brachyury (T; Herrmann, 1992) in the primitive streak was also normal in Dkk1 mutant embryos (Figures 3E and 3F), and trunk formation appeared unaffected. At E8.5, transcripts of Six3, an anterior-most marker (Oliver et al., 1995), were not detectable in Dkk1 mutant embryos (Figures 3I and 3J), further confirming that inductive events leading to forebrain development were defective. We conclude that Dkk1 gene activity is required for forebrain induction. Roles of Mouse Dkk1 in Head and Limb Development 425 Figure 1. Gene Targeting at the Dkk1 Locus and the Anterior Head Phenotype of Dkk1⫺/⫺ Mutant Embryos (A) Partial restriction maps of the wild-type Dkk1 locus, the targeting vector, and the disrupted Dkk1 allele. The entire coding region of the Dkk1 allele including the intervening intronic sequences was replaced with the neomycin (neo) cassette. Lines at the bottom indicate the expected size of ApaI-generated fragments in the wild-type (11 kb) and the disrupted (4.4 kb) allele when detected with a 5⬘ external genomic probe. Ap, ApaI; H3, HindIII; Xb, XbaI. (B) Southern blot analysis of ApaI-digested genomic DNA isolated from yolk sacs of wildtype (⫹/⫹), heterozygous (⫹/⫺), and homozygous mutant (⫺/⫺) embryos at E14.5. (C–E) Scanning electron micrographs of E9.5 wild-type (C) and mutant embryos (D and E) in the lateral (C and D) and frontal (E) orientation. The heart (h) appears normal in the mutant, but structures anterior to the second branchial arch (b) and to the otic pit (arrowheads) including the optic eminence, the mandibular and maxillary components of the first branchial arch, and the nasal process are absent in the mutant embryo. (F) Lateral view of E10.5 control (⫹/⫹) and mutant (⫺/⫺) embryos. Note the abnormal morphology of the brain region anterior to the fourth ventricle (arrows) in the mutant embryo. (G) Lateral view of E17.5 control (⫹/⫹) and mutant (⫺/⫺) embryos. At this stage, the mutant embryo is slightly smaller than the control embryo and has a severely truncated head. The kink in the tail of the mutant embryo has not been reproducibly observed. (H) Higher magnification view of the head region of the mutant embryo (different embryo than shown in [G]), showing the lack of craniofacial structures anterior to the external ear process (e). (I–K) Bone (red) and cartilage (blue) staining of E17.5 heads of wild-type (I) and mutant (J and K) embryos. Lateral (J) and top (K) views show that the Dkk1⫺/⫺ mutant embryos lack all bones anterior to the parietal bone (p), which is smaller in the mutant than in the control embryo. c1, c1 vertebrae; e, exoccipital bone; f, frontal bone; i, interparietal bone; mn, mandible; mx, maxillary bone; n, nasal bone; p, parietal bone; pm, premaxillary bone; s, supraoccipital bone. The scale bar represents 300 m in (C–E); 1 mm in (F); 2.5 mm in (G); 1 mm in (H); and 2 mm in (I–K). Localization of Dkk1 Function during Gastrulation Dkk1 may function in head formation by influencing the inductive properties of the extraembryonic AVE, the embryonic AME, or both. Recent data suggest that head induction in the mouse embryo requires not only a functional AVE but also the node and its AME derivative (Bachiller et al., 2000; Shawlot et al., 1999; Tam and Steiner, 1999). Dkk1 is expressed in the AVE as well as the AME (Glinka et al., 1998; Pearce et al., 1999). In order to determine which of these two tissues contains the headinducing activity, we performed a chimeric analysis based on the finding that ES cells injected into host blastocysts almost exclusively contribute to embryonic tissue, while the host cells predominantly contribute to the extraembryonic lineage (Beddington and Robertson, 1989). Using this technique, we were able to generate chimeric embryos composed mainly of Dkk1-expressing epiblast cells developing within the confines of a Dkk1⫺/⫺ visceral endoderm. In order to determine whether Dkk1 gene function is essential in the AVE, Rosa-26 ES cells carrying the wild-type Dkk1 alleles and expressing a -galactosidase transgene were injected into blastocysts obtained from Dkk⫹/⫺ intercrosses. The genotype of the host embryos was determined by PCR analysis of cells from the endodermal layer of the yolk sac. Eight embryos were unequivocally identified as being derived from ES cell-injected Dkk⫺/⫺ host embryos. Each of these was phenotypically indistinguishable from chimeric Dkk⫹/⫹ or Dkk⫹/⫺ host embryos, irrespective of the fact that, based on staining with X-gal (5-bromo-4-chloro-3-indolyl--D-galactopyranoside), the admixture of wild-type ES cell derivatives was high in some but only modest in others (Figure 4). Developmental Cell 426 Figure 2. Histological Analysis of the Head Phenotype in Dkk1⫺/⫺ Mutant Embryos, Using Tissue-Specific Molecular Markers Sagittal sections of E11.5 wild-type (A, C, E, G, and I) or mutant (B, D, F, H, and J) embryos were either stained with hematoxylin and eosin (A and B) or were hybridized with tissuespecific probes (C–J). (A and B) The abnormal morphology of the mutant brain ([B] compared with [A]) suggests an apparent lack of the telencephalon and the diencephalon in the Dkk1 mutant embryo. (C and D) Expression of Shh in the ventral midline of the mecencephalon and myelencephalon of the mutant embryo (D) is similar to that observed in the wild-type embryo (C). (E and F) Expression of En2 in the midbrain/ hindbrain boundary is also present in the Dkk1 mutant embryo (F). (G and H) In addition to the normal expression of Otx2 in the midbrain region (G), the Dkk1 mutant embryos show a signal in the dorsal myelencephalon (H). (I and J) Expression of BF1 is normally restricted to the forebrain region (I) and is completely absent in the Dkk1 mutant embryo (J). d, diencephalon; m, mesencephalon; my, myelencephalon; t, telencephalon. The scale bar represents 250 m. We conclude that Dkk1 function in the AVE is not essential for proper anterior morphogenesis. Expression Patterns of Dkk1 in the Developing Vertebrate Limb Bud Our functional assessment of the role of Dkk1 in limb development began with the analysis of the spatio-temporal pattern of expression of its mRNA during limb bud outgrowth in both the mouse and the chick embryo, thereby extending earlier studies (Grotewold et al., 1999; Monaghan et al., 1999). At the initial stages of mouse limb bud development, we detected Dkk1 transcripts in the lateral plate mesoderm of the prospective limb bud cells (data not shown). A few hours later, at around E9.5, Dkk1 expression is noted in the distal mesenchymal and ectodermal cells of the nascent limb buds (Figure 5A). At E10.5, Dkk1 transcripts begin to be excluded from the distal-most mesenchymal cells of the limb bud and become restricted to two patches located at the anterior and posterior sides of the limb bud (Figure 5B). At this developmental stage, expression is also seen in the apical ectodermal ridge (AER). This expression is maintained for a couple of days (Figure 5C), and at E13.5 when the paw plates appear, Dkk1 expression becomes confined to the interdigital space. An almost identical pattern of expression of Dkk1 was detected during chick limb bud outgrowth (Figures 6A–6D). The anterior and posterior patches of Dkk1 expression at the limb bud stages, as well as the interdigital areas, correlate well with regions known to undergo programmed cell death. Roles of Mouse Dkk1 in Head and Limb Development 427 Figure 4. Chimeric Analysis for Dkk1 Gene Function Figure 3. Whole-Mount Analysis of Forebrain Markers in Dkk1⫺/⫺ Mutant Embryos (A) Whole-mount in situ hybridization with Hesx1 probe at the late streak stage (E7.5) in a wild-type embryo marks the endoderm underlying the future forebrain and overlying epiblast in which the forebrain is forming. The arrows point to the embryonic-extraembryonic junction. (B) Dkk1 mutant embryo showing the absence of Hesx1 expression. (C–F) The pattern of expression of Otx2 (C and D) and Brachyury (T) (E and F) at E7.5 appears normal in Dkk1 mutant embryos. (G–J) Forebrain tissue expressing Hesx1 (G and H) and Six3 (I and J) is completely absent in Dkk1 mutant embryos at E8.5. The scale bar represents 100 m. Analysis of the Limb Phenotype in Dkk1 Null Mutant Mouse Embryos In addition to the anterior head phenotype described earlier, Dkk1⫺/⫺ mice also show fore- and hindlimb malformations. As shown in Figures 5E–5G, Dkk1⫺/⫺ limb buds display a slightly thickened AER as compared to stage-matched wild-type controls. Later phenotypes range from a slight widening of the limb buds to fusion of the distal-most limb elements and the appearance of ectopic preaxial and postaxial digits. The most common phenotype observed in Dkk1⫺/⫺ limbs was a fusion of digits II and III (Figures 5J–5M) and the appearance of an extra digit I and/or an extra digit V (Figures 5N–5Q). Dkk1 Chimeric embryos were stained with X-gal in order to detect the contribution of injected Dkk⫹/⫹ cells in the host embryos of Dkk⫺/⫺ genotype. X-gal staining detects the expression of the -galactosidase gene driven by the Rosa-26 promoter, and acts as a visible marker for the donor cells (Zambrowicz et al., 1997). (A) X-gal-stained chimeric embryo in which the genotype of the host embryo is Dkk⫹/⫹. (B) X-gal-stained chimeric embryo in which the genotype of the host embryo is Dkk⫺/⫺. The embryo proper contains a high percentage (90%–95%) of injected Dkk⫹/⫹ (blue) cells and the head phenotype is rescued. (C and D) Hematoxylin and eosin-stained sagittal sections of the embryos shown in (A) and (B), respectively. No difference in the histology of the two sections is detected. f, forebrain; m, midbrain; h, hindbrain. The scale bar represents 500 m. In order to determine the molecular basis of the defects in limb outgrowth caused by the lack of Dkk1 in the limb, we analyzed the expression of several genes known to be involved in limb growth and patterning. We focused our attention on genes that have been associated with distal outgrowth and programmed cell death, since these processes appeared to be primarily affected in Dkk1⫺/⫺ limb buds. We analyzed, among others, the expression pattern of several members of the HoxD gene family (hoxD9–13), Shh, and Bmp2, 4, and 7. No deviation from their normal pattern of expression was detected. By contrast, the expression of Fgf8, a marker for the AER, was very much increased, and its expression domain was expanded toward both the dorsal and ventral ectoderm (Figures 5R and 5S). Since constitutive activation of FGF signaling has been shown to correlate with the appearance of syndactyly (Yu et al., 2000), the increase in Fgf8 transcripts observed in the distal part of Developmental Cell 428 Figure 5. Absence of Dkk1 Alters Mouse Limb Outgrowth Whole-mount in situ hybridization of Dkk1 at limb outgrowth. (A) Transcripts for Dkk1 are detected initially in the distal part of the developing limb bud in both the mesenchyme and the ectoderm. (B and C) Subsequently, between E10 and E12, Dkk1 mRNA is confined toward the anterior and posterior areas of cell death. (D) Later on, Dkk1 mRNA is detected in the interdigital areas of the developing limb. (E–I) Scanning electron microscopy images of wild-type and Dkk1⫺/⫺ limbs. At stages E10.5 to E11.5, the AER of Dkk1⫺/⫺ limbs appears to be slightly enlarged (arrows in [F] and [G]). (H and I) At later stages, the appearance of ectopic digits, as well as fusion of digits, is observed in Dkk1⫺/⫺ limb buds. (J–Q) Alcian blue staining and whole mount of wild-type and Dkk1⫺/⫺ limbs showing the normal cartilage pattern in (J) (forelimb) and (N) (hindlimb). The interdigital soft tissue in the distal part of Dkk1⫺/⫺ limbs is not clearly separated (arrows in [K] and [O]). Additional digits both preaxially and postaxially develop in Dkk1⫺/⫺ limb buds (arrows in [L], [M], [P], and [Q]). (R and S) Ffg8 expression is more intense and wider in the mutant when compared to the wild-type limb buds (arrows in [S]). (T and U) On the contrary, the expression of Msx1 is downregulated in the Dkk1⫺/⫺ limbs (arrow in [U]). the Dkk1⫺/⫺ limbs could be a trigger for the fusion of some limb elements. The appearance of ectopic pre- and postaxial digit elements could be due to alterations in the genetic program that regulates cell death during limb outgrowth. Thus, we analyzed the expression of Msx1, a gene involved in programmed cell death (Gañán et al., 1998). Msx1 is expressed at early limb bud stages in the most distal cells, as well as in the anterior and posterior regions of the limb bud that will undergo cell death (Gañán et al., 1998). In Dkk1⫺/⫺ limb buds, reduced levels of Msx1 transcripts were observed, both in the distal and the posterior and anterior limb bud cells (Figures 5T and 5U). The reduced expression of a gene marker involved in cell death may indicate that the ectopic digit elements observed in Dkk1⫺/⫺ limbs result from an alteration in the genetic mechanisms that regulate programmed cell death. Overexpression of Dkk1 Inhibits Limb Outgrowth in the Chick Embryo To further explore the role of Dkk1 during vertebrate limb outgrowth, we constructed an adenoviral vector encoding the full-length chick Dkk1 gene and infected presumptive fore- and hindlimb regions of stage 10 chick embryos. Limb buds infected with this viral construct showed a deletion of distal limb tissue with the AER broken up into two or more domains (33% of injected embryos; Figures 6E and 6F). In more severe cases, the AER was totally absent, resulting in a very reduced limb bud (25% of injected embryos; Figure 6G). Whole-mount in situ hybridization to detect the integrity of the AER was performed using the Fgf8 probe. As shown in Figure 6H, the expression domain of Fgf8 was either interrupted or totally absent (data not shown). When the injected embryos were allowed to develop further (up to 13 days), we observed alterations in cartilage patterning that correlated well with the alterations Roles of Mouse Dkk1 in Head and Limb Development 429 Figure 6. Ectopic Dkk1 Expression Alters Chick Limb Outgrowth (A–D) Similar to the mouse limb bud, chick Dkk1 transcripts are expressed initially in the distal part of the nascent limb bud (A) and later on become confined to the posterior and anterior areas of cell death, as well as to the AER (B, C, and D). (E–K) Phenotypes caused by the infection of stage 10 presumptive limb regions with an adenovirus expressing Dkk1. Ectopic expression of Dkk1 causes the splitting of the AER (E and F) and/or a severely reduced limb bud (G). The expression of Fgf8 is downregulated in the Ad-Dkk1-infected limbs (H). (I) Day 12 infected embryo that developed from Ad-Dkk1-infected forelimb. Note the vestigial limb (arrow). (J and K) Alcian green staining of similarly infected embryos showing truncation of the medial and distal elements of the forelimb (arrow in [J]) and hindlimb (arrow in [K]). (L and M) A bead soaked in BMP2 protein is able to induce the expression of both Msx1 (arrow in [L]) and Dkk1 (arrow in [M]) within 1 hr of implantation. (N) Injection of Ad-Dkk1 at stage 10 in the presumptive limb region results 2 days later in the downregulation of BMP2 expression in both the AER and the mesenchyme cells (arrow). in the distal limb bud areas indicated above. In 35% of the injected embryos, limbs were severely truncated and lacked the medial and distal limb elements in both foreand hindlimbs (Figures 6I–6K). From these results, we conclude that restriction of Dkk1 expression to the posterior and anterior regions of the early limb bud is essential for normal limb outgrowth. Previous reports have demonstrated that the BMP signaling pathway is involved in controlling both cell death and limb outgrowth (reviewed by Chen and Zhao, 1998). The observation that both an absence and an excess of Dkk1 apparently correlate with an alteration in the apoptotic cell process that takes place during the sculpturing of the vertebrate limb suggested a link between Dkk1 and BMP signaling pathways. Among the different BMP family members, Bmp2 displays a pattern of expression very similar to that of Dkk1 during limb outgrowth. Thus, we decided to explore a possible relationship between BMP signaling and Dkk1 activity by implanting beads soaked in BMP2 in the developing chick limb bud. As shown in Figure 6L, BMP2 is able to upregulate Msx1 (a direct target of BMP signaling; Gañán et al., 1996) and Dkk1 (Figure 6M) within 1 hr after bead implantation. Conversely, overexpression of Dkk1 downregulated the expression of BMP2 in both mesenchymal cells and in the AER (Figure 6N). Altogether, these results indicate not only that Dkk1 deregulation is incompatible with normal limb development but that they also unveil the existence of a link between Dkk1 and BMP activities during limb outgrowth. Discussion A Role for Dkk1 in Head Development Our study has shown that ablation of Dkk1 function in the mouse results in the severe truncation of forebrain and cephalic neural crest-derived head tissues. This finding provides direct genetic evidence that Dkk1, a Wnt antagonist, plays an essential role in vertebrate head development and supports previous notions that inhibition of Wnt- and BMP-mediated signaling pathways is essential for proper anterior neural development. De Robertis and colleagues have recently shown that mouse embryos carrying null mutations in the genes encoding the BMP antagonists Noggin and Chordin fail to maintain a functional AVE and display forebrain defects (Bachiller et al., 2000). Consistent with the posteriorizing role of Wnt family members, ectopic expression of Wnt8 causes the truncation of anterior neuroectoderm in transgenic mice (Pöpperl et al., 1997), and the forced expression of the Wnt antagonist Frzb1 reduces the formation of posterior mesoderm (Borello et al., 1999). Furthermore, the inactivation of Tcf3, a repressor of Wnt target genes in zebrafish, results in microcephaly (Kim et al., 2000). Dkk1 expression in the early embryo is first noted in cells corresponding to a region of the early gastrulating embryo (at E6.5) where the anterior visceral endoderm (AVE) abuts the epiblast (Glinka et al., 1998; Pearce et al., 1999; Zakin et al., 2000). However, no morphological defect can be detected in the Dkk1⫺/⫺ embryos prior to the headfold stage. At the molecular level, the absence of Hesx1 gene expression in the prospective anterior neuroectodermal (ANE) cells of late streak mutant embryos is the earliest defect detected in our study. The complete absence of Hesx1 at E7.5 suggests that Dkk1 function is required for the proper expression of this gene in the AVE as well. Hesx1 expression in the ANE is required for forebrain development (Martinez-Barbera et al., 2000). Expression of Six3, another gene activated in the ANE at late streak stages (Oliver et al., 1995), is Developmental Cell 430 also undetectable in Dkk1⫺/⫺ mutants. Hesx1 and Six3 are the earliest known ANE markers in the mouse. Expression of these genes in the ANE of a wild-type embryo starts at E7.5 and continues through early somite stages (Oliver et al., 1995; Hermesz et al., 1996; Thomas and Beddington, 1996). In the Hesx1⫺/⫺ mutant, Six3 expression in the ANE appears normal at late streak stages but is reduced at the early somite stage (Martinez-Barbera et al., 2000). Therefore, the absence of Six3 expression at the early somite stage of our Dkk1⫺/⫺ mutants may be a consequence of the loss of Hesx1 expression. Proper anterior positioning of the early Dkk1-expressing AVE cells appears to be controlled by Otx2 (Zakin et al., 2000; Perea-Gomez et al., 2001), a marker which seems unaffected in our Dkk1⫺/⫺ mutants. The severe anterior phenotype of Otx2⫺/⫺ embryos suggests that Otx2 is a key factor in the head developmental process (Acampora et al., 1995; Matsuo et al., 1995). Since Dkk1 acts downstream of Otx2, it most likely mediates forebrain induction pathways activated by Otx2. Forebrain patterning in the mouse is initiated by the inductive activity of the AVE and subsequently requires the function of the node-derived AME (Ang et al., 1994; Thomas and Beddington, 1996; Tam and Steiner, 1999; Shawlot et al., 1999). We find that chimeric embryos, largely composed of Dkk1⫹/⫹ epiblast cells developing within the confines of a Dkk1⫺/⫺ visceral endoderm, display a seemingly normal anterior morphology at E9.5. This shows that Dkk1 gene expression in the AVE is not required for head induction. Notably, a recent study has demonstrated that the function of Hesx1, the gene that is essential for forebrain development and acts downstream of Dkk1, is dispensable in the AVE (MartinezBarbera et al., 2000). Thus, cells of the elongating axial mesendoderm are the likely source of the Dkk1 signal that mediates the rescue of head organization in the chimera. This conclusion is based on studies in other vertebrates showing that Dkk1 function in the AME is required for head development. In Xenopus, injection of Dkk1 mRNA, together with a BMP inhibitor, is able to induce the complete duplication of head structures. It is believed that the head duplication resulted from an ectopic expression of prechordal plate (AME) markers, such as Xhex and Xgsc (Kazanskaya et al., 2000; Hashimoto et al., 2000). Our work identifies Dkk1, a secreted factor associated with the role of AME, in early rostral specification of the mouse embryo. We propose that Hesx1 function in the ANE is mediated through Dkk1, which is secreted by adjacent AME. Dkk1 fits the role of a ligand that interacts with a receptor to protect the prospective anterior neuroectoderm via Wnt inhibition from caudalizing effects of the node (Foley et al., 2000), thereby exerting an early and indispensable function in head induction. Interestingly, Dkk1 has recently been shown to bind the Wnt coreceptor LRP6 (LDL receptor-related protein 6), thus most likely repressing type I Wnt signaling (Mao et al., 2001; Semënov et al., 2001). We do not yet know which type I Wnt protein is mediating negative control of head formation in the mouse. However, in Xenopus, Wnt8 has been implicated in this process (Hoppler et al., 1996). Our study clearly shows that the ablation of Dkk1 function affects not only brain development but also that of surrounding head structures. Various degrees of head truncation have also been observed in mice carrying null mutations in other factors expressed during early stages of head induction including Hesx1, Otx, and Lim1 (Dattani et al., 1998; Acampora et al., 1995; Matsuo et al., 1995; Ang et al., 1996). This suggests that there may be a coordinated morphogenetic response of neural and nonneural precursor cells to head-inducing signals. A Role for Dkk1 in Limb Development Our results also demonstrate a key role for Dkk1 in vertebrate limb development. Its pattern of expression in mouse and chick limb buds suggests specific activities in both the mesenchymal and AER components of the limb. In the mesenchyme of the early limb bud, the expression domains of Dkk1 overlap with the anterior and posterior necrotic zones. In the later stages of limb development, expression of Dkk1 becomes restricted to the interdigital mesenchyme, which also undergoes programmed cell death (PCD; reviewed by Chen and Zhao, 1998). The involvement of Dkk1 in the control of PCD is supported by the phenotype of the developing Dkk1⫺/⫺ forelimbs. We observed the fusion of digits and ectopic anterior and posterior digits similar to those seen in mice mutant for other genes involved in the control of PCD in the limb, notably members of the Bmp gene family (Luo et al., 1995; Katagiri et al., 1998). Thus, the lack of Dkk1 function in the anterior and posterior margins of the limb mesenchyme may interfere with PCD in these areas, leading to the development of extra digits either pre- or postaxially. Interference with the process of PCD in the interdigital mesenchyme could also explain digit fusion. Consistent with these interpretations, the general level of expression of Msx1, a member of a gene family directly involved in the control of PCD (reviewed by Bendall and Abate-Shen, 2000), is severely reduced in Dkk1⫺/⫺ limb buds. Moreover, crossregulation between the products of Bmp and Dkk1 genes may contribute to fine tuning the extent of PCD in the limb mesenchyme, since Dkk1 appears to be a target of BMP signaling. However, we also found that an excess of Dkk1 downregulates Bmp expression. This suggests that an adequate balance between the activities of BMPs and Dkk1 is a prerequisite for adequate spatial and temporal control of PCD in the mesenchyme. The mechanism by which Dkk1 regulates PCD in the limb mesenchyme remains to be determined. The lack of Dkk1 activity in the AER results in a pronounced expansion of the Fgf8 domain, and most likely of the AER itself. Conversely, we observed that overexpression of Dkk1 in the chick limb bud interferes with AER induction and Fgf8 expression, severely perturbing limb outgrowth. We can interpret these phenotypes in the light of recent results on the role of a Wnt gene, Wnt3a, in AER induction and Fgf8 activation in the chick limb bud. During the initial stages of limb bud induction, the activation of Wnt3a expression in the pre-AER ectodermal cells precedes Fgf8 activation and AER induction (Kengaku et al., 1998; Kawakami et al., 2001). Since Dkk1 encodes a protein which functions as an extracellular antagonist of Wnt ligands, the lack of the Dkk1 antagonist might result in the hyperactivity of the pathway triggered by Wnt3A in the AER, thus leading to an excess Roles of Mouse Dkk1 in Head and Limb Development 431 of Fgf8 expression. This underscores the importance of a tight regulation of the amount of Wnt signaling that operates in the AER. As we show here, Dkk1 appears to play an important role in this regulation. The most distal mesenchymal cells of the limb bud (progress zone) are kept in a proliferative state by the AER and give rise to the most distal limb structures (Summerbell et al., 1973). The ectodermal expansion of Fgf8 transcripts in Dkk⫺/⫺ limb bud cells is likely to result in prolonged proliferation that may delay differentiation of distal mesenchymal cells during subsequent stages of limb development. Constitutive activation of FGFs can cause digit fusion (Yu et al., 2000), and selective ablation of Fgf8 expression during limb development can lead to hypoplasia or loss of digits (Lewandoski et al., 2000; Moon and Capecchi, 2000). Together, these findings suggest that Fgf8 activation in response to Dkk1 functional ablation may also be responsible for the observed Dkk1⫺/⫺ limb phenotype. Therefore, two different mechanisms, reduced PCD and increased FGF8 activity, may cooperate to increase cell proliferation and promote digit fusion and the formation of extra digits in the distal part of the developing Dkk1⫺/⫺ limbs. In conclusion, we demonstrate that the Wnt antagonist Dkk1 plays an essential role during mammalian development. Our findings show that Dkk1 mediates inductive interactions between AME and the anterior neural ectoderm essential for forebrain development in mice. Furthermore, we show that a balance between Dkk1 and BMP signaling is important for regulating programmed cell death in sculpturing the vertebrate limb. In general, results of this study emphasize the necessity for the inhibition of Wnt signaling for proper head specification and limb morphogenesis. Experimental Procedures Generation of Dkk1⫺/⫺ Mutant Mice The targeting vector was constructed by replacing the entire coding region of Dkk1 with a PGK-NEO cassette, preserving 3.8 kb (5⬘) and 2.4 kb (3⬘) of flanking homologous sequences and using the thymidine kinase gene for double selection. The final targeting vector was linearized at a unique NotI site before the transfection of ES cells. Transfected ES cells were subjected to double selection. Targeted disruption of the Dkk1 gene via homologous recombination was confirmed by Southern blot analysis. Two independently derived recombinant ES cell lines were injected into blastocysts to generate chimeric mice. Chimeric males were mated with C57BL/6 females to produce Dkk1⫹/⫺ animals, which were intercrossed to produce offspring for analysis. Genotyping DNA was extracted from extraembryonic membranes of E9.0 and older embryos. For E7.5 to E8.5 embryos, whole embryos were digested overnight upon the completion of in situ analysis and photography (Lowe and Kuehn, 2000). The genotype of the embryos was determined by polymerase chain reaction using the following primers: Dkk1 wild-type allele primers 5⬘-GGGAGCCTGAGTA TAAAGGC-3⬘ and 5⬘-AAGAGTCTGGTACTTGTTCC-3⬘; and NEO primers 5⬘-CTTGGGTGGAGAGGCTATTC-3⬘ and 5⬘-AGGTGAGAT GACAGGAGATC-3⬘, which yielded bands of 416 and 280 bp, respectively. Histology and In Situ Hybridization Cartilage and bones were stained with alcian blue and alizarin red (Wallin et al., 1994). Embryos were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned at 5 m. Sections were either stained with hematoxylin and eosin for histological analysis or were hybridized to 35S-labeled antisense riboprobes specific to Shh (Echelard et al., 1993), En2 (Joyner and Martin, 1987), Otx2 (Simeone et al., 1993), and BF1 (Tao and Lai, 1992) as described (Robinson et al., 1991). In situ hybridization to whole embryos was performed as described (Lowe and Kuehn, 2000; Saga et al., 1996) using antisense riboprobes to Hesx1 (Thomas and Beddington, 1996), Otx2 (Simeone et al., 1993), Brachyury (T; Herrmann, 1992), and Six3 (Oliver et al., 1995). For marker analysis in the limb, embryos were processed for whole-mount in situ hybridization as described (Rodriguez-Esteban et al., 1997). Alcian blue cartilage staining was performed according to Vogel et al. (1996). The entire open reading frames of both mouse and chick Dkk1 were used for riboprobe synthesis. The rest of the probes are described by Capdevila et al. (1999), and details may be provided upon request. Chimeric Analysis Chimeric embryos were generated by injecting 20–30 ES cells (Rosa26, Dkk1⫹/⫹) into blastocysts obtained from Dkk1⫹/⫺ intercrosses. Injected blastocysts were transferred to pseudopregnant females and recovered 7–8 days later, corresponding to embryonic days E9.5 and E10.5, respectively. The genotype of the host embryos was determined by PCR analysis of DNA from visceral yolk sac endoderm isolated by pancreatin/ trypsin digestion (Hogan et al., 1994). Embryos were fixed in 1% paraformaldehyde, and -galactosidase expression was visualized by X-gal staining of wholemount embryos using standard protocol (Hogan et al., 1994). Isolation of Chick Dkk1 Chick Dkk1 was isolated by screening a stage 21–23 HH chick cDNA library with a probe derived from the mouse Dkk1 gene using standard conditions (42% formamide, 4⫻ SSC, 0.1% SDS, and 100 g/ ml denatured salmon sperm DNA at 42⬚C). The open reading frame of chick Dkk1 has been deposited with GenBank. Viral Infection and Bead Implantation Chicken embryos (either MacIntire Poultry, San Diego, CA or SPAFAS, Norwich, CT) were infected with adenovirus Dkk1 in the wing or leg primordia at stages 8–10 as previously described (Capdevila et al., 1999). Adenoviruses were produced as described (Miyake et al., 1996). Beads were soaked in BMP2 (provided by Genetic Institute) as described (Capdevila et al., 1999). After different incubation times, embryos were fixed in 4% paraformaldehyde and dehydrated in methanol. After evaluation under a dissecting microscope for possible alterations, embryos were stored at ⫺20⬚C until processed for in situ hybridization. Acknowledgments We thank A. Tomac, A. Agulnick, and Y. Zhao for helpful discussions and suggestions; M. Yarolin and A. Schindler for assistance in tissue preparation; and R. Beddington, G. Oliver, and E. De Robertis for materials. A.G. and C.N. are supported by the Deutsche Forschungsgemeinschaft. C.R.-E., T.T., and J.C.I.B. are supported by the NIH and the G. Harold and Leila Y. Mathers Charitable Foundation. Received April 11, 2001; revised July 20, 2001. References Acampora, D., Mazan, S., Lallemand, Y., Avantaggiato, V., Maury, M., Simeone, A., and Brulet, P. (1995). Forebrain and midbrain regions are deleted in Otx2⫺/⫺ mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121, 3279–3290. Ang, S.L., Conlon, R.A., Jin, O., and Rossant, J. (1994). Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120, 2979–2989. Ang, S.L., Jin, O., Rhinn, M., Daigle, N., Stevenson, L., and Rossant, J. (1996). A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122, 243–252. Developmental Cell 432 Bachiller, D., Klingensmith, J., Kemp, C., Belo, J.A., Anderson, R.M., May, S.R., McMahon, J.A., McMahon, A.P., Harland, R.M., Rossant, J., and De Robertis, E.M. (2000). The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403, 658–661. Beddington, R.S., and Robertson, E.J. (1989). An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development 105, 733–737. Beddington, R.S., and Robertson, E.J. (1998). Anterior patterning in mouse. Trends Genet. 14, 277–284. Belo, J.A., Bachiller, D., Agius, E., Kemp, C., Borges, A.C., Marques, S., Piccolo, S., and De Robertis, E.M. (2000). Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis 26, 265–270. Bendall, A.J., and Abate-Shen, C. (2000). Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 247, 17–31. Bhanot, P., Brink, M., Harryman Samos, C., Hsieh, J.-C., Wang, Y., Macke, J.P., Andrew, D., Nathans, J., and Nusse, R. (1996). A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230. Borello, U., Coletta, M., Tajbakhsh, S., Leyns, L., De Robertis, E.M., Buckingham, M., and Cossu, G. (1999). Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development 126, 4247–4255. Bradley, L., Wainstock, D., and Sive, H. (1996). Positive and negative signals modulate formation of the Xenopus cement gland. Development 122, 2739–2750. Cadigan, K.M., and Nusse, R.M. (1997). Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305. C. (1997). Head induction by simultaneous repression of BMP and Wnt signalling in Xenopus. Nature 389, 517–519. Glinka, A., Wu, W., Delius, H., Monaghan, P.A., Blumenstock, C., and Niehrs, C. (1998). Dickkopf1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362. Grotewold, L., Theil, T., and Rüther, U. (1999). Expression pattern of DKK1 during mouse limb development. Mech. Dev. 89, 151–153. Hashimoto, H., Itoh, M., Yamanaka, Y., Yamashita, S., Shimizu, T., Solnica-Krezel, L., Hibi, M., and Hirano, T. (2000). Zebrafish DKK1 functions in forebrain specification and axial mesendoderm formation. Dev. Biol. 217, 138–152. Hermesz, E., Mackem, S., and Mohan, K.A. (1996). Rpx: a novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate and Rathke’s pouch of the mouse embryo. Development 122, 41–52. Herrmann, B.G. (1992). Action of the Brachyury gene in mouse embryogenesis. Ciba Found. Symp. 165, 78–86. Hogan, B., Beddington, R., Costantini, F., and Lacy, E. (1994). Isolating extraembryonic membranes. In Manipulating the Mouse Embryo. A Laboratory Manual (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 160–162. Hoppler, S., Brown, J.D., and Moon, R.T. (1996). Expression of a dominant-negative wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10, 2805–2817. Hsieh, J.C., Kodjabachian, L., Rebbert, M.L., Rattner, A., Smallwood, P.M., Samos, C.H., Nusse, R., Dawid, I.B., and Nathans, J. (1999). A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398, 431–436. Camus, A., and Tam, P.P. (1999). The organizer of the gastrulating mouse embryo. Curr. Top. Dev. Biol. 45, 117–153. Huelsken, J., Vogel, R., Brinkmann, V., Erdmann, B., Birchmeier, C., and Birchmeier, W. (2000). Requirement for -catenin in anteriorposterior axis formation in mice. J. Cell Biol. 148, 567–578. Capdevila, J., Tsukui, T., Rodriguez-Esteban, C., Zappavigna, V., and Izpisúa Belmonte, J.C. (1999). Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol. Cell 4, 839–849. Joyner, A.L., and Martin, G.R. (1987). En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1, 29–38. Chen, Y., and Zhao, X. (1998). Shaping limbs by apoptosis. J. Exp. Zool. 282, 691–702. Katagiri, T., Boorla, S., Frendo, J.L., Hogan, B.L., and Karsenty, G. (1998). Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev. Genet. 22, 340–348. Christian, J.L., and Moon, R.T. (1993). Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 7, 13–28. Kawakami, Y., Capdevila, J., Buscher, D., Itoh, T., Esteban, C.R., and Belmonte, J.C. (2001). Wnt signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104, 891–900. Dattani, M.T., Martinez-Barbera, J.P., Thomas, P.Q., Brickman, J.M., Gupta, R., Martensson, I.L., Toresson, H., Fox, M., Wales, J.K., Hindmarsh, P.C., et al. (1998). Mutations in the homeobox gene HESX1/ Hesx1 associated with septo-optic dysplasia in human and mouse. Nat. Genet. 19, 125–133. Echelard,Y., Epstein, D.J., St-Jacques, B., Shen, L., Mohler, J., McMahon, J.A., and McMahon, A.P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430. Ellies, D.L., Church, V., Francis-West, P., and Lumsden, A. (2000). The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development 127, 5285–5295. Foley, A.C., Skromne, I., and Stern, C.D. (2000). Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127, 3839–3854. Gañán, Y., Macias, D., Duterque-Coquillaud, M., Ros, M., and Hurle, J. (1996). Role of TGFBs and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development 122, 2349–2357. Gañán, Y., Macias, D., Basco, R.D., Merino, R., and Hurle, J.M. (1998). Morphological diversity of the avian foot is related with the pattern of Msx gene expression in the developing autopod. Dev. Biol. 196, 33–41. Glinka, A., Wu, W., Onichtchouk, D., Blumenstock, C., and Niehrs, Kazanskaya, O., Glinka, A., and Niehrs, C. (2000). The role of Xenopus Dickkopf1 in prechordal plate specification and neural patterning. Development 127, 4981–4992. Kengaku, M., Capdevila, J., Rodriguez-Esteban, C., De La Peña, J., Johnson, R.L., Belmonte, J.C., and Tabin, C.J. (1998). Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science 280, 1274–1277. Kim, C.H., Oda, T., Itoh, M., Jiang, D., Artinger, K.B., Chandrasekharappa, S.C., Driever, W., and Chitnis, A.B. (2000). Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916. Krupnik, V.E., Sharp, J.D., Jiang, C., Robison, K., Chickering, T.W., Amaravadi, L., Brown, D.E., Guyot, D., Mays, G., Leiby, K., et al. (1999). Functional and structural diversity of the human Dickkopf gene family. Gene 238, 301–313. Lewandoski, M., Sun, X., and Martin, G.R. (2000). Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 26, 460–463. Leyns, L., Bouwmeester, T., Kim, S.-H., Piccolo, S., and De Robertis, E.M. (1997). Frzb-1 is a secreted antagonist of wnt-signals expressed in the Spemann organizer. Cell 88, 747–756. Liu, P., Wakamiya, M., Shea, M.J., Albrecht, U., Behringer, R.R., and Bradley, A. (1999). Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 22, 361–365. Roles of Mouse Dkk1 in Head and Limb Development 433 Lowe, L.A., and Kuehn, M.R. (2000). Whole mount in-situ hybridization to study gene expression during mouse development. Methods Mol. Biol. 137, 125–137. Luo, G., Hofmann, C., Bronckers, A.L., Sohocki, M., Bradley, A., and Karsenty, G. (1995). BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820. Mao, B., Wu, W., Li, Y., Hoppe, D., Stannek, P., Glinka, A., and Niehrs, C. (2001). LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325. Martinez-Barbera, J.P., Rodriguez, T.A., and Beddington, R.S. (2000). The homeobox gene Hesx1 is required in the anterior neural ectoderm for normal forebrain formation. Dev. Biol. 223, 422–430. Marvin, M.J., Di Rocco, G., Gardiner, A., Bush, S.M., and Lassar, A.B. (2001). Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 15, 316–327. Matsuo, I., Kuratani, S., Kimura, C., Takeda, N., and Aizawa, S. (1995). Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 9, 2646–2658. McGrew, L.L., Hoppler, S., and Moon, R.T. (1997). Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech. Dev 69, 105–114. Miyake, S., Makimura, M., Kanegae, Y., Harada, S., Sato, Y., Takamori, K., Tokuda, C., and Saito, I. (1996). Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl. Acad. Sci. USA 93, 1320–1324. Monaghan, A.P., Kioschis, P., Wu, W., Zuniga, A., Bock, D., Poustka, A., Delius, H., and Niehrs, C. (1999). Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech. Dev. 87, 45–56. Moon, A.M., and Capecchi, M.R. (2000). Fgf8 is required for outgrowth and patterning of the limbs. Nat. Genet. 26, 455–459. Moon, R.T., Brown, J.D., and Torres, M. (1997a). Wnts modulate cell fate and behavior during vertebrate development. Trends Genet. 13, 157–162. Moon, R.T., Brown, J.D., Yang-Snyder, J.A., and Miller, J.R. (1997b). Structurally related receptors and antagonists compete for secreted Wnt ligands. Cell 88, 725–728. Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W., and Roder, J.C. (1993). Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90, 8424–8428. Niehrs, C. (1999). Head in the WNT: the molecular nature of Spemann’s head organizer. Trends Genet. 15, 314–319. Oliver, G., Mailhos, A., Wehr, R., Copeland, N.G., Jenkins, N.A., and Gruss, P. (1995). Six3, a murine homologue of the Sine Oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045– 4055. Pearce, J.J., Penny, G., and Rossant, J. (1999). A mouse Cerberus/ Dan-related gene family. Dev. Biol. 209, 98–110. Perea-Gomez, A., Lawson, K.A., Rhinn, M., Zakin, L., Brulet, P., Mazan, S., and Ang, S.L. (2001). Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development 128, 753–765. Piccolo, S., Agius, E., Leyns, L., Bhattacharya, S., Grunz, H., Bouwmeester, T., and De Robertis, E.M. (1999). The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397, 707–710. Pöpperl, H., Schmidt, C., Wilson, V., Hume, C.R., Dodd, J., Krumlauf, R., and Beddington, R.S.P. (1997). Misexpression of Cwnt8c in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development 124, 2997–3005. Rattner, A., Hsieh, J.-C., Smallwood, P.M., Gilbert, D., Copeland, N.G., Jenkins, N.A., and Nathans, J. (1997). A family of secreted proteins contains homology to the cysteine-rich ligand binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 94, 2859–2863. Robinson, G.W., Wray, S., and Mahon, K.A. (1991). Spatially restricted expression of a member of a new family of murine Distalless homeobox genes in the developing forebrain. New Biol. 3, 1183– 1194. Rodriguez-Esteban, C., Schwabe, J.W.R., De La Peña, J., Foys, B., Eshelman, B., and Izpisúa Belmonte, J.C. (1997). Radical fringe positions the apical ectodermal ridge at the dorsoventral boundary of the vertebrate limb. Nature 386, 360–366. Saga, Y., Hata, N., Kobayashi, S., Magnuson, T., Seldin, M., and Taketo, M.M. (1996). MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122, 2769–2778. Schneider, V.A., and Mercola, M. (1999). Spatially distinct head and heart inducers within the Xenopus organizer region. Curr. Biol. 9, 800–809. Schneider, V.A., and Mercola, M. (2001). Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 15, 304–315. Semënov, M.V., Tamai, K., Brott, B.K., Kuhl, M., Sokol, S., and He, X. (2001). Head inducer Dickkopf-1 is a ligand for Wnt co-receptor LRP6. Curr. Biol. 11, 951–961. Shawlot, W., Wakamiya, M., Kwan, K.M., Kania, A., Jessell, T.M., and Behringer, R.R. (1999). Lim1 is required in both primitive streakderived tissues and visceral endoderm for head formation in the mouse. Development 126, 4925–4932. Shawlot, W., Min Deng, J., Wakamiya, M., and Behringer, R.R. (2000). The Cerberus-related gene, Cer1, is not essential for mouse head formation. Genesis 26, 253–258. Shinya, M., Eschbach, C., Clark, M., Lehrach, H., and Furutani-Seiki, M. (2000). Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech Dev. 98, 3–17. Simeone, A., Acampora, D., Mallamaci, A., Stornaiuolo, A., D’Apice, M.R., Nigro, V., and Boncinelli, E. (1993). A vertebrate gene related to orthodenticle contains a homeodomain of the Bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 12, 2735–2747. Stanley, E.G., Biben, C., Allison, J., Hartley, L., Wicks, I.P., Campbell, I.K., McKinley, M., Barnett, L., Koentgen, F., Robb, L., and Harvey, R.P. (2000). Targeted insertion of a LacZ reporter gene into the mouse Cer1 locus reveals complex and dynamic expression during embryogenesis. Genesis 26, 259–264. Summerbell, D., Lewis, J.H., and Wolpert, L. (1973). Positional information in chick limb morphogenesis. Nature 244, 492–496. Tam, P.P., and Steiner, K.A. (1999). Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development 126, 5171–5179. Tao, W., and Lai, E. (1992). Telencephalon-restricted expression of Bf-1, a new member of the HNF-3/fork Head gene family, in the developing rat brain. Neuron 8, 957–966. Thomas, P., and Beddington, R. (1996). Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr. Biol. 6, 1487–1496. Vogel, A., Rodriguez, C., and Izpisúa Belmonte, J.C. (1996). Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development 2, 1737–1750. Wallin, J., Wilting, J., Koseki, H., Fritsch, R., Christ, B., and Balling, R. (1994). The role of Pax-1 in axial skeleton development. Development 120, 1109–1121. Wang, S., Krinks, M., Lin, K., Luyten, F.P., and Moos, M. (1997). Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88, 757–766. Wodarz, A., and Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88. Wu, W., Glinka, A., Delius, H., and Niehrs, C. (2000). Mutual antagonism between Dickkopf1 and -2 regulates Wnt/-catenin signalling. Curr. Biol. 10, 1611–1614. Yu, K., Herr, A.B., Waksman, G., and Ornitz, D.M. (2000). Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl. Acad. Sci. USA 97, 14536–14541. Developmental Cell 434 Zakin, L., Reversade, B., Virlon, B., Rusniok, C., Glaser, P., Elalouf, J.M., and Brulet, P. (2000). Gene expression profiles in normal and Otx2⫺/⫺ early gastrulating mouse embryos. Proc. Natl. Acad. Sci. USA 97, 14388–14393. Zambrowicz, B.P., Imamoto, A., Fiering, S., Herzenberg, L.A., Kerr, W.G., and Soriano, P. (1997). Disruption of overlapping transcripts in the ROSA  geo 26 gene trap strain leads to widespread expression of -galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94, 3789–3794. Zoltewicz, J.S., and Gerhart, J.C. (1997). The Spemann organizer of Xenopus is patterned along its anteroposterior axis at the earliest gastrula stage. Dev. Biol. 192, 482–491. Accession Numbers The Genbank accession number for the open reading frame of chick Dkk1 reported in this paper is AY049017.