* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Uric acid
Fatty acid metabolism wikipedia , lookup
Lipid signaling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Genetic code wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Point mutation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Butyric acid wikipedia , lookup
Peptide synthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Biochemistry wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
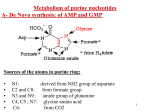
Metabolism of Nucleotides 核 苷 酸 代 谢 Jiao Li Department of biochemistry and molecular biology 65986142 [email protected] Is nucleic acid dietarily essential? Nucleic acid health product What makes up of our DNA and RNA? Learning Objectives After completion of this section, you should be able to : 1. Indicate the source of each atom in purine and pyrimidine ring. 2. Describe anabolism (de novo and salvage) and catabolism of purine and pyrimidine. 3. Outline the sequence of reactions in de novo and salvage and the conversion of IMP to AMP or GMP, and UMP to CTP or TMP. 4. Understand the mechanism of anticancer drugs and allopurinol in treating gout. Outline of the section 1. Nomenclature and structure of nucleotide. 2. Biosynthesis of purine and pyrimidine 3. Feedback inhibition and cross regulation in purine and pyrimidine synthesis. 4. Catabolism of purine and pyrimidine. 5. Clinical significance. Section 1 Nomenclature and structure of nucleotide 1. (Deoxy)Ribonucleotide is basic subunit of DNA or RNA (1) Nomenclature and structure of bases purines Adenine Guanine pyrimidines Cytosine Uracil Thymine U is only present in RNA T is present in DNA These are purine and pyrimidine bases. (2) Types of sugar Ribose is only present in RNA. Deoxyribose is only present in DNA. (3) Deoxyribonucleotide in DNA Base Adenine Guanine Cytosine Thymine Deoxynucleoside deoxyadenosine deoxyguanosine deoxythymidine deoxycytidine Deoxyribonucleotide adenosine monophosphate (AMP) deoxyadenosine triphosphate (dATP) (4) Ribonucleotide in RNA Base Adenine Guanine Cytosine Uracil Nucleotide adenosine guanosine Nucleoside uridine cytidine Nucleotide adenosine monophosphate (AMP) deoxyadenosine triphosphate (dATP) Section 2 Biosynthesis of purine and pyrimidine Overview of dietary nucleotide metabolism Dietary nucleoprotein protein Gastric acid Nuclei acid(RNA or DNA) (deoxy)ribonuclease from pancreas nucleotide Nucleotidase from intestinal endothelium nucleoside phosphate nucleoside phosphorylase from intestinal endothelium Uric acid base inside intestinal endothelium (deoxy)ribose We do not need to eat purines and pyrimidines in our diet because we have ways to make them. Our body has two ways of making purine and pyrimidine nucleotides: De novo synthesis and the salvage pathway. New or recycled. We recycle more than we make new. 1. De novo synthesis of purine nucleotide •Source of nitrogen and carbon atoms of the purine ring CO2 glycine Aspartate N5,N10methenyl- tetrahydrofolate N10 formyl tetrahydrofolate glutamine (amide nitrogen) • De novo synthesis of purine nucleotide includes two major processes: (1) Synthesis from PRPP to IMP (2) Conversion of IMP to GMP and AMP. • Major site: liver • De novo synthesis of purine is weak in brain and erythrocyte. Overview of de novo pathway for synthesis of purine Key step1 Key step2 IMP (1) Synthesis from PRPP to IMP 1st step Key enzyme The 1st step in the pathway is the synthesis of PRPP by PRPP synthetase. This step is regulated by feedback inhibition. Function of PRPP PRPP is the source of the ribose sugar and 5’ phosphate found in purine nucleotides. 2nd step—— Base synthesis starts PRPP amidotransferase catalyzes this step. This is the first committed step in de novo synthesis of purines. This step adds an amine from glutamine to PRPP. 3rd step GAR synthetase The ribose sugar and the phosphate are indicated by P-Ribose because this part does not change in any of the steps. Glycine is the source of 2 carbons and a nitrogen of the purine ring. Glycinamide ribosyl-5-phosphate 4th step N5,N10- methenyl tetrahydrofolate Formyltransferase Formylglycinamide ribosyl-5-phosphate In this step one carbon is donated by N5,N10- methenyl tetrahydrofolate. 5th step Synthetase Formylglycinamidine ribosyl-5-phosphate In this step glutamine donates an amine. 6th step ATP The ring closes to make the 5 membered ring. Synthetase ADP+Pi Aminoimidazole ribosyl-5-phosphate 7th step Carboxylase Aminoimidazole carboxylate ribosyl-5-phosphate A carbon atom is donated by carbon dioxide. 8th step synthetase Aminoimidazole succinyl carboxamide ribosyl-5-phosphate Aspartate is added. Only the nitrogen of aspartate will remain in the final purine ring. 9th step Most of the aspartate atoms are removed. Only the nitrogen of aspartate remains. Adenylosuccinase Aminoimidazole carboxamide ribosyl-5-phosphte 10th step Formyltransferase Formaminoimidazole carboxamide ribosyl-5-phosphate A carbon atom is donated by N10-formyltetrahydrofolate. 11th (last) step Cyclohydrolase In the last step the ring closes to make IMP. You are not expected to memorize pathways! De novo synthesis of purine is on the basis of PRPP by addition of simple molecules step by step. IMP is an important intermediate in de novo synthesis of purine. IMP AMP GMP (2) Conversion of IMP to GMP and AMP fumarate Asp Adenylosuccinate ① Adenylosuccinate synthetase ③ IMP dehydrogenase ② Adenylosuccinase ④ Transamidinase 2. Salvage pathway of purine synthesis What are salvage pathways? Salvage pathways recover bases and nucleosides by converting them to nucleotides. (1) Save energy (2) Provide purines to erythrocyte and brain(no de novo) How do salvage pathways proceed ? (1) Base is converted to nucleotide A to adenosine monophosphate G to guanosine monophosphate How do salvage pathways proceed ? (2) Nucleoside is converted to nucleotide Adenosine (dAdenosine, dGuanosine) Adenosine kinase ATP ADP AMP (dAMP, dGMP) Relationship of nucleotides, nucleosides, nucleobases All compounds in this box are nucleotides Nucleobases in this box Nucleosides in this box Case 1: Lesch Nyhan syndrome HGPRT is the "salvage enzyme" for the purines, failure of this enzyme has two results: *Cell breakdown products cannot be reused, and are therefore degraded. This gives rise to increased uric acid. *The de novo pathway is stimulated due to an excess of PRPP. X-linked genetic disease with: Neurologic disfunction; behavial disturbance; high uric acid Abnormal metabolism leads to disease The CNS involvement is not caused by uric acid. The brain has an extremely low capacity for de novo purine biosynthesis, so a complete absence of the salvage enzyme damages the brain by depriving it of purine nucleotides. 3. De novo synthesis of pyrimidine •Source of nitrogen and carbon atoms of the pyrimidine ring CO2 • De novo synthesis of pyrimidine nucleotide includes two major processes: (1) Synthesis from PRPP to UMP (2) Conversion of UMP to CTP and TMP. (1) Synthesis from PRPP to UMP Key step UMP (2) Conversion of UMP to CTP and TMP UMP 4. Salvage pathway of pyrimidine synthesis Salvage pathway of pyrimidine transferase Pyrimidine + PRPP nucleoside+ PPi Uridine kinase Uridine + ATP UMP +ADP Thymidine kinase Thymidine + ATP dTMP +ADP Overview of synthesis for pyrimidine Uridine kinase 5. Formation of deoxyribonucleotides by ribonucleotide reductase Ribonucleotide reductase makes DNA precursors Ribonucleotides, the precursors for RNA, are made first in the de novo pathway. Deoxyribonucleotides, the precursors for DNA, are made later and on the basis of NDP. Ribonucleotide reductase reduces all 4 ribonucleotides to deoxyribonucleotides NDP(A,C,G,U) dNDP kinase dNDP + ATP dNTP + ADP Section 3 Feedback inhibition and cross regulation in purine and pyrimidine synthesis (1) Regulation of purine synthesis When we speak of regulation of purine synthesis what we mean is (a) regulation of IMP synthesis, and (b) regulation of synthesis of GMP and AMP from IMP α-D-Ribose 5-phosphate PRPP activation Phosphoribosylamine Feedback inhibition Feedback inhibition Cross-regulation (2) Regulation of pyrimidine synthesis PRPP(+) In bacteria synthesis is activated by ATP and inhibited by CTP. In mammals synthesis is activated by PRPP and inhibited by UTP. UTP(-) Setion 4 Catabolism of purine and pyrimidine (1) Catabolism of purines The end product of purine catabolism in man and birds is uric acid. Uric acid is formed primarily in the liver and is excreted by the kidneys into the urine. (1) Catabolism of purines Xanthine oxidase Guanine deaminase Adenosine deaminase Xanthine oxidase Nucleoside phosporylase Uric acid (not very soluble) Case 2: Gout Uric acid and urate salts are not very soluble. Crystals of sodium urate precipitate in joints, especially the big toe, causing severe pain. Sodium urate crystals can damage the kidneys, causing death. 3 out of 1000 people have gout. Causes of gout Overproduction of purine nucleotides, leading to excessive uric acid synthesis. Inadequate salvage of purines. Impaired uric acid excretion through the kidneys. Three enzymes defect lead to gout Catabolism of purines Xanthine oxidase Guanine deaminase Adenosine deaminase Xanthine oxidase Nucleoside phosporylase Uric acid (not very soluble) Mechanism of gout The unneeded excess purine nucleotides are degraded to uric acid. Uric acid is not very soluble. The excess uric acid cannot stay in solution. It precipitates, causing gout. Gout or death can be caused by impaired uric acid secretion Chemotherapy for cancer can overload the kidneys with degraded purines after the death of cells. Kidney stones may form and lead to death due to kidney failure. Verify the dose of chemotherapy drugs Treatment 1 of gout Allopurinol inhibits xanthine oxidase, thus reducing the amount of uric acid made. Limit intake of foods that are rich in nucleic acids, for example meat. Limit alcohol intake because alcohol affects kidney function. Catabolism of purines Xanthine oxidase (d) Guanine deaminase Adenosine deaminase Xanthine oxidase Nucleoside phosporylase Uric acid (not very soluble) Gouty toe After allopurinol Treatment 2 of gout Another way to treat gout is with probenecid, a drug that increases renal secretion of uric acid. Probenecid Probenecid Uric acid × Colchicine CH 3O H N C CH 3O OCH 3 CH 3 O O OCH 3 COLCHICINE Toxic natural product from Colchicum autumnale (Meadow Saffron). Cochicine relieves the pain of gout. It is anti-inflammatory. Catabolism of purines Xanthine oxidase (d) Guanine deaminase Adenosine deaminase Xanthine oxidase Nucleoside phosporylase Uric acid (not very soluble) Case 3: Combined immunodeficiency Excess dATP caused by adenosine deaminase deficiency inhibits ribonucleotide reductase and leads to immunodeficiency disease. Catabolism of purines dATP Xanthine oxidase (d) Guanine deaminase Adenosine deaminase Xanthine oxidase Nucleoside phosporylase Adenosine deaminase deficiency • Clinical characteristics: inactive immune system and therefore inability to fight infections. Death occurs in early childhood. When ribonucleotide reductase is inhibited by dATP there are not enough nucleotides for DNA synthesis. This makes it impossible for white cells to proliferate rapidly. White cells must proliferate for an immune response to occur. First gene therapy disease (2) Catabolism of pyrimidines deaminase Very water soluble Section 5 Clinical significance We have described several diseases associated with purine metabolism Gout Lesch-Nyhan syndrome Combined immunodeficiency disease Some purine or pyrimidine analogs are used as anticancer drugs 5-fluorouracil(5-FU) 6-mercaptopurine(6-MP) Methotrexate (1) 5-FdUMP inhibit thymidylate synthase Breast cancer and colon cancer are treated with 5-fluorouracil. F 5-Fluorouracil 5-fluorouracil is an analog of thymine F 5-fluorouracil uracil thymine 5-fluorouracil is an inactive precursor drug. It must be converted to a nucleotide by the salvage pathway before it is an active drug. Transferase; kinase Active drug form FdUMP inhibits thymidylate synthase. As a result, DNA synthesis slows down. FdUMP Proliferation of cancer cells slows down. (2) 6-MP inhibit salvage of purine TIMP inhibit de novo synthesis of purine (—) hypoxanthine (I) HGPRT 6-mercaptopurine (6-MP) 6-thioinosine-5-monophosphate (TIMP) (—) PRPP-glutamyl amidotransferase (3)Methotrexate inhibt dihydrofolate reductase Both compounds are similar and bind to dihydrofolate reductase. Dihydrofolate reductase activity is essential for production of dTMP Dihydrofolate reductase dTMP Dihydrofolate reductase regenerates methylene FH4, the donor of the CH3 group in dTMP. Methotrexate methotrexate Methotrexate inhibits dihydrofolate reductase. Cells cannot produce adequate amounts of dTMP for DNA synthesis. In this way methotrexate inhibits proliferation of cancer cells. Summary 1. Source of each atom in purine and pyrimidine is from simple molecule. 2. Tow major way of biosynthesis of purine and pyrimidine- De novo and salvage pathway. 3. Feedback inhibition and cross regulation in purine and pyrimidine synthesis. 4. End product of purine catabolism is uric acid. 5. Gout, Lesh-Nyhan syndrome and Combined immunodeficiency syndrome are associated with abnormal metabolism of purine. Study question: 1. What is the mechanism of action of allopurinol for treatment of gout? a. Allopurinol solubilizes uric acid crystals by binding to them. b. Allopurinol increases excretion of uric acid by the kidneys. c. Allopurinol inhibits the enzyme that makes uric acid. d. Allopurinol decreases the inflammatory response.