* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2 ATP`s - Madeira City Schools
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
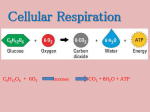
I. Introduction to Cellular Respiration A. Respiration vs. Cellular Respiration B. When you breath you exchange O2 and CO2; Cellular Respiration, you use O2 to “burn” sugar which gives off CO2 as waste. C. Formula: C6H12O6 + 6O2 ---> 6CO2 + 6H2O + ATP 1. Only get 40% of glucose’s potential energy. Rest is converted to heat. 2. Organic molecule not always glucose (can be fats and protein too) II. The Basics A. Cellular respiration (CR) breaks glucose in a series of steps. B. The relocation of electrons releases energy stored in food. This energy is used to make ATP. C. Redox reaction (oxidation and reduction reaction) 1. Oxidation = loss of electrons from one substance Loss of electrons. Loss of energy. Loss of Hydrogens from Carbons. 2. Reduction = addition of electrons to another substance Gain of electrons. Gain of energy. Gain of Hydrogens to Carbons. 3. Electron transfers require both a donor and an acceptor, these reactions always go together. Rs D. NAD+ (nicotinamide adenine dinulcleotide) a coenzyme, functions as an oxidizing agent (something that accepts electrons from a substance being oxidized). This is an “electron carrier compound” (shuttles electrons within the cell) 1. Enzymes known as dehydrogenases remove a pair of H atoms from the substrate and/or cytoplasm. 2. The enzyme delivers 2 electrons and 1 proton to its coenzyme (NAD+) 3. The other proton is released as a H+ ion. 4. NAD+ is reduced to NADH 5. NADH is stored energy that can be tapped to make ATP when the electrons complete their “fall” from NADH to Oxygen (a very good oxidizing agent) a. This “fall” occurs in the electron transport chain (part 3 of CR) E. An overview: CellularRespiration occurs in 3 metabolic stages 1. Glycolysis a. occurs in the cytoplasm b. break down glucose into 2 molecules of pyruvate c. ATP made here by substrate-level phosphorylation 2. Krebs cycle a. occurs in the mitochondrial matrix b. breaks down a derivative of pyruvate to carbon dioxide c. ATP made here by substrate-level phosphorylation 3. Electron transport chain a. occurs in the inner membrane of the mitochondria of Euks and in the plasma membrane in Proks b. uses products from above reaction to get energy so ATP can be made. c. Mode of ATP synthesis is Chemiosmosis (coupling mechanism for oxidative phosphorylation) Glycolysis Krebs Cycle Electron Transport Chain Small amounts of ATP are made by “Substrate level phosphorylation” – an enzyme transfers a phosphate group from a substrate molecule to ADP. The substrate molecule is a glucose “intermediate” as it is broken down. III. The Process of Cellular Respiration A. Glycolysis: Prepartory Phase 1. Glucose enters cytoplasm of cell. 2. Glucose is phosphorylated with the help of enzyme hexokinase. a. this substrate-enzyme complex traps glucose in cell b. makes glucose more reactive 3. Glucose-6 phosphate is rearranged by an enzyme to Fructose-6 phosphate (an isomer) 4. Fructose-6 phosphate is phosphorylated with help from enzyme phosphofructokinase (now it is ready to split) 5. With the help from aldolase Fructose-1,6 biphosphate splits. 6. Dihydroxyacetone phosphate and Glyceraldehyde phosphate (G3P) are isomers that result from split. B. Glycolysis: The Energy Payoff Phase 1. Dihydroxyacetone phosphate rearranges with the help from enzyme isomerase to become Glyceraldehyde phosphate 2. Both Glyceraldehyde phosphates are oxidized a. NAD+ ---> NADH + H+ b. exergonic reaction…the energy is used to attach an inorganic phosphate group (in the cytoplasm) to both of the oxidized substrates 3. The Phosphate groups on both the Glyceraldehyde phosphates are removed and attached to ADPs to make 2 ATPs a. This process is called “substrate phosphorylation” 4. The two 3-Phosphoglycerate molecules go through an enzymatic reaction where the remaining phosphate group is relocated on the molecule to prepare it for the next reaction. 5. The two 2-Phosphoglycerate molecules bond with enzyme enolase (double bonding) causing water to be extracted. This causes the phosphate to be unstable. 6. The resulting molecules, phosphoenolpyruvate (PEP), lose their unstable phosphate to ADP, resulting in 2 more ATP forming from “substrate phosphorylation” 7. After losing the phosphate, you now have 2 molecules of Pyruvate and glycolysis is complete. Energy Harvest Phase Glycolysis Energy Yield: Use 2 ATP molecules to start Gain 4 ATP molecules from substrate phosphoylation Therefore, net ATP gain is 2 ATP’s Also gain 2 NADH that will transfer the electrons gained to the electron transport chain to assist in making more ATPs. QuickTime™ and a Cinepak decompressor are needed to see this picture. Net Energy Gain ---> 2 NADH C. Grooming Pyruvate prior to the Krebs cycle (formation of acetyl CoA) 1. Pyruvate enters mitochondrial matrix from the cytoplasm with the help from a transport protein imbedded in the mitochondrial membrane 2. A carboxyl group is removed as a CO2 molecule which diffuses out of the cell. 3. Remaining 2C fragment is oxidized while NAD+ is reduced to NADH 4. Coenzyme A (a Sulfur containing compound derived from vitamin B) joins with the 2 carbon molecule to yield Acetyl CoA. a. this is a high energy fuel molecule b. the coenzyme activates the acetyl group for the first reaction in the Krebs cycle. D. Krebs Cycle Named for by German-British Scientist Hans Krebs--1930’s 1. Coenzyme A helps the acetyl part enter the cycle, then it splits off and is recycled. a. Acetyl (2C) bonds with oxaloacetate (4C) b. resulting molecule is a 6 Carbon molecule, Citric acid. 2. Water is added and taken away causing Citric acid to change to its isomer, Isocitrate 3. Isocitrate loses a carboxyl group as CO2 4. The new 5C molecule is oxidized while NAD+ is reduced to NADH. 5. The 5 carbon molecule, alpha-ketoglutarate, loses a carboxyl as CO2 and the resulting molecule is oxidized while NAD+ is reduced to NADH. a. This step is catalyzed by an enzyme similar to the one that converts pyruvate to acetyl CoA. 6. The 4 Carbon molecule, Succinate, bonds with Coenzme A 7. Succinyl CoA goes through substrate phosphorylation a. CoA is displaced by a phosphate group b. Phosphate group is transferred to GDP (similar to ADP) to form GTP c. GTP transfers phosphate to ADP to form ATP 8. Succinate is oxidized while FAD is reduced to FADH2 a. (flavin adenine dinucleotide, derived from riboflavin …a B vitamin) 9. 4 Carbon molecule is rearranged and oxidized to become Oxaloacetate while NAD+ is reduced to NADH. a. This molecule joins an acetyl group to make citric acid and the process continues. Net Energy Yield---> 2 ATP 6 NADH 2 FADH2 QuickTime™ and a Cinepak decompressor are needed to see this picture. Energy accumulated so far: 2 ATP from Glycolysis 2 ATP from Krebs Cycle 4 ATP 2 NADH from Glycolysis 10 NADH 2 NADH from Pyruvate Grooming 2 FADH2 6 NADH from Krebs Cycle 2 FADH2 from Krebs Cycle NADH and FADH2 are electron “escorts” to the electron transport chain. As the electrons go through the chain, the energy released is used to make ATP. Making ATP this way is called “oxidative phosphorylation”. E. Electron Transport Chain 1. The “chain” is a collection of molecules embedded in the inner membrane of the mitochondria. a. Cristae increases this surface area (structure/function) 2. Most components of the chain are proteins. a. “Prosthetic groups” (similar to cofactors) are tightly bound to these proteins. During electron transport, they alternate between reduced and oxidized states as they accept and donate electrons. 3. Electrons removed during glycolysis and Krebs are transferred by NADH to the first molecule of the chain called Flavoprotein (FMN) 4. Flavoprotein is oxidized as it passes the electrons to an iron-sulfur protein (Fe-S) 5. The iron-sulfur protein is oxidized as it passes the electrons to a lipid (the only carrier not a protein) called Ubiquinone (Q) 6. Most of the remaining electron carriers between Q and Oxygen are proteins called Cytochromes (cyt). a. Their prosthetic group is a heme group. b. It transfers electrons, not oxygen. c. The last cytochrome (cyt a3) passes its electrons to Oxygen, which also picks up a pair of hydrogen ions from the surrounding solution to form water. 7. The chain does not make ATP directly. Its function is to break a large energy drop into a series of smaller steps so energy is released in manageable amounts. 8. How does the mitochondria couple electron transport and energy release to making ATP? Chemiosmosis Order of electron carriers: FMN Fe-S Q Cyt b Fe-S Cyt c1 Cyt c Cyt a Cyt a3 F. Chemiosmosis 1. Definition: Using the energy of H+ gradient across a membrane to phosphorylate ADP (“Oxidative Phosphorylation”) to make ATP. 2. The flow of electrons through the chain produces energy that is used to pump H+ ions across the membrane (from matrix to intermembrane space). a. When certain carriers within the chain transfer electrons, H+ ions are also taken up and released back into the surrounding solution. b. The carriers are arranged in the membrane so that H+ is accepted from the matrix and deposited in the intermembrane space. c. The gradient that results is referred to as a proton-motive force. d. How does ETC “pump” H+ ions? certain members of the ETC accept and release H+ ions along with electons whereas other carries transport only electrons. the above members are spatially arranged in the membrane in such a way that H+ ions are accepted from the matrix and deposited in the intermembrane space. 3. How ATP is made from this gradient: a. ATP Synthase is the enzyme that makes ATP. It is located in the inner membrane of the mitochondria. Made of three main parts: a cylinder within the inner mitochondrial membrane a knob protruding into the mitochondrial matrix an internal rod connecting the two. b. ATP Synthase uses the proton motive force (the energy from the gradient) to make ATP. c. When H+ ions flow through the cylinder, with their gradient, they cause the cylinder and the attached rod to rotate. d. The spinning rod causes the knob to change conformation. This conformation activates catalytic sites where ADP and inorganic phosphate combine to make ATP. GLYCOLYSIS: 2 ATP’S KREBS CYCLE: 2 ATP’S ELECTRON TRANSPORT 10 NADH 2 FADH2 TOTAL 30 ATP’S * 4 ATP’S 38 ATP’S * average ATP yield per NADH is probably between 2 and 3 * The mitochondrial inner membrane is impermeable to NADH, so the 2 electrons of NADH from glycolysis get in by one of two ways…Either NAD+ or FAD (which ever one will determine the total number of ATP’s IV. Fermentation A. Definitions 1. Fermentation = A mechanism of oxidizing organic fuel (glucose) and generating ATP WITHOUT the help of oxygen 2. Aerobic= oxygen is present 3. Anaerobic= oxygen is NOT present 4. Oxidizing Agent= the substance that gains electrons B. The Basics of Fermentation 1. The process of fermentation is an extension of glycolysis 2. Generates energy by substrate-level phosphorylation 3. NAD+ is the oxidizing agent in fermentation a. NAD+ is also the oxidizing agent of glycolysis b. Needs to be sufficient NAD+ to accept e- during the oxidation step of glycolysis c. When e- are added, NAD+ becomes NADH 4. Electrons are transferred from NADH to pyruvate (end product of glycolysis) 5. This process produces 2 ATP C. Two types of fermentation 1. Alcohol Fermentation= pyruvate is converted into ethanol (ethyl alcohol) a. Step 1: Carbon dioxide is released from pyruvate -> becomes a 2-carbon compound (acetaldehyde) b. Step 2: Acetaldehyde is reduced by NADH to ethanol c. Examples: yeast (does brewing and wine making) and some bacteria Alcohol Fermentation 2. Lactic Acid Fermentation= pyruvate is converted to lactate a. No release of carbon dioxide b. Pyruvate is directly reduced by NADH to form lactic acid (3 carbon molecule) c. Human muscle cells make lactic acid when oxygen is scarce (ATP production out paces muscle oxygen supply from the blood) d. Lactate is converted back to pyruvate in liver cells e. Examples: fungi, bacteria used in the production of cheese and yogurt Lactic Acid Fermentation Microbes produce acetone or methanol D. Fermentation vs. Respiration 1. Similarities: a.Use glycolysis to oxidize glucose (or other organic molecules) to pyruvate b.Net of 2 ATP by substrate phosphorylation c.NAD+ is the oxidizing agent (accepts e-) and needs to be regenerated *how it is regenerated is different 2. Differences: a.Fermentation = Anaerobic; Respiration =Aerobic b.Fermentation final e- acceptor= pyruvate; Respiration final e- acceptor = oxygen c.Fermentation generates 2 ATP Respiration generates 38 ATP V. Alternatives for glucose A. Glucose does not always need to be the molecule broken down in respiration or fermentation 1. Starch and Glycogen a.can be hydrolyzed to become glucose 2. Proteins a.must be broken down into amino acids first b.enzymes convert aa to intermediates of glycolysis and Krebs cycle c.amine group must be removed (deamination) in the form of ammonia, urea or other waste d. proteins yield about the same amount of energy as carbohydrates 3. Fats a. Breaks down into glycerol (3-C cmpd) and fatty acids b. Glycerol is converted into G3P and fed into the glycolytic pathway c. Beta oxidation= breaks down fatty acids to a 2-carbon fragment to enter Krebs as acetyl CoA d. Two times as much ATP in a gram of fat than in carbs *Not all organic molecules (such as glucose, proteins and fats, etc.) go into making ATP…they use ATP for other purposes -carbon skeletons -proteins for the body VI. Feedback Mechanisms A. Metabolism is regulated by supply and demand 1. Controlled by enzymes along key locations in glycolysis and Krebs cycle a.Feedback inhibition (negative feedback) = end product of an anabolic pathway inhibits the enzyme that catalyzes an early step of the pathway 3. ATP concentration is low, respiration speeds up 4. ATP concentration is high, respiration slows down (organic molecules can be used elsewhere)