* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Mechanosensitive channels wikipedia , lookup
Cell encapsulation wikipedia , lookup
Lipid bilayer wikipedia , lookup
Cytokinesis wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Extracellular matrix wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
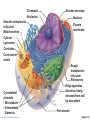
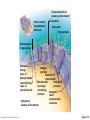
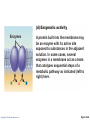
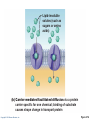
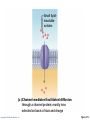
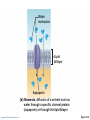
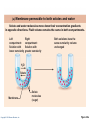
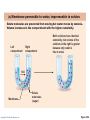
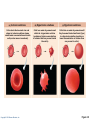
PowerPoint® Lecture Slides prepared by Janice Meeking, Mount Royal College CHAPTER 3 Cells: The Living Units: Part A Copyright © 2010 Pearson Education, Inc. Cell Theory • The cell is the smallest structural and functional living unit • Organismal functions depend on individual and collective cell functions • Biochemical activities of cells are dictated by their specific subcellular structures • Continuity of life has a cellular basis Copyright © 2010 Pearson Education, Inc. Developmental Aspects of Cells • All cells of the body contain the same DNA but are not identical • Chemical signals in the embryo channel cells into specific developmental pathways by turning some genes off • Development of specific and distinctive features in cells is called cell differentiation • Elimination of excess, injured, or aged cells occurs through programmed rapid cell death (apoptosis) followed by phagocytosis Copyright © 2010 Pearson Education, Inc. Theories of Cell Aging • Wear and tear theory: Little chemical insults and free radicals have cumulative effects • Immune system disorders: Autoimmune responses and progressive weakening of the immune response • Genetic theory: Cessation of mitosis and cell aging are programmed into genes. Telomeres (strings of nucleotides on the ends of chromosomes) may determine the number of times a cell can divide. Copyright © 2010 Pearson Education, Inc. Generalized Cell • All cells have some common structures and functions • Human cells have three basic parts: • Plasma membrane—flexible outer boundary • Cytoplasm—intracellular fluid containing organelles • Nucleus—control center Copyright © 2010 Pearson Education, Inc. Chromatin Nucleolus Nuclear envelope Nucleus Smooth endoplasmic reticulum Mitochondrion Cytosol Lysosome Centrioles Centrosome matrix Cytoskeletal elements • Microtubule • Intermediate filaments Copyright © 2010 Pearson Education, Inc. Plasma membrane Rough endoplasmic reticulum Ribosomes Golgi apparatus Secretion being released from cell by exocytosis Peroxisome Figure 3.2 Plasma Membrane • Bimolecular layer of lipids and proteins in a constantly changing fluid mosaic • Plays a dynamic role in cellular activity • Separates intracellular fluid (ICF) from extracellular fluid (ECF) • Interstitial fluid (IF) = ECF that surrounds cells Copyright © 2010 Pearson Education, Inc. Extracellular Materials • Body fluids (interstitial fluid, blood plasma, and cerebrospinal fluid) • Cellular secretions (intestinal and gastric fluids, saliva, mucus, and serous fluids) • Extracellular matrix (abundant jellylike mesh containing proteins and polysaccharides in contact with cells) Copyright © 2010 Pearson Education, Inc. Extracellular fluid (watery environment) Polar head of phospholipid molecule Cholesterol Glycolipid Glycoprotein Carbohydrate of glycocalyx Outwardfacing layer of phospholipids Integral proteins Filament of cytoskeleton Peripheral Bimolecular Inward-facing proteins lipid layer layer of containing phospholipids Nonpolar proteins tail of phospholipid Cytoplasm molecule (watery environment) Copyright © 2010 Pearson Education, Inc. Figure 3.3 Membrane Lipids • 75% phospholipids (lipid bilayer) • Phosphate heads: polar and hydrophilic • Fatty acid tails: nonpolar and hydrophobic (Review Fig. 2.16b) • 5% glycolipids • Lipids with polar sugar groups on outer membrane surface • 20% cholesterol • Increases membrane stability and fluidity Copyright © 2010 Pearson Education, Inc. Functions of Membrane Proteins 1. Transport 2. Receptors for signal transduction 3. Attachment to cytoskeleton and extracellular matrix Copyright © 2010 Pearson Education, Inc. (a) Transport A protein (left) that spans the membrane may provide a hydrophilic channel across the membrane that is selective for a particular solute. Some transport proteins (right) hydrolyze ATP as an energy source to actively pump substances across the membrane. Copyright © 2010 Pearson Education, Inc. Figure 3.4a Signal Receptor Copyright © 2010 Pearson Education, Inc. (b) Receptors for signal transduction A membrane protein exposed to the outside of the cell may have a binding site with a specific shape that fits the shape of a chemical messenger, such as a hormone. The external signal may cause a change in shape in the protein that initiates a chain of chemical reactions in the cell. Figure 3.4b (c) Attachment to the cytoskeleton and extracellular matrix (ECM) Elements of the cytoskeleton (cell’s internal supports) and the extracellular matrix (fibers and other substances outside the cell) may be anchored to membrane proteins, which help maintain cell shape and fix the location of certain membrane proteins. Others play a role in cell movement or bind adjacent cells together. Copyright © 2010 Pearson Education, Inc. Figure 3.4c Functions of Membrane Proteins 4. Enzymatic activity 5. Intercellular joining 6. Cell-cell recognition Copyright © 2010 Pearson Education, Inc. (d) Enzymatic activity Enzymes Copyright © 2010 Pearson Education, Inc. A protein built into the membrane may be an enzyme with its active site exposed to substances in the adjacent solution. In some cases, several enzymes in a membrane act as a team that catalyzes sequential steps of a metabolic pathway as indicated (left to right) here. Figure 3.4d (e) Intercellular joining Membrane proteins of adjacent cells may be hooked together in various kinds of intercellular junctions. Some membrane proteins (CAMs) of this group provide temporary binding sites that guide cell migration and other cell-to-cell interactions. CAMs Copyright © 2010 Pearson Education, Inc. Figure 3.4e (f) Cell-cell recognition Some glycoproteins (proteins bonded to short chains of sugars) serve as identification tags that are specifically recognized by other cells. Glycoprotein Copyright © 2010 Pearson Education, Inc. Figure 3.4f Membrane Transport • Plasma membranes are selectively permeable • Some molecules easily pass through the membrane; others do not Copyright © 2010 Pearson Education, Inc. Types of Membrane Transport • Passive processes • No cellular energy (ATP) required • Substance moves down its concentration gradient • Active processes • Energy (ATP) required • Occurs only in living cell membranes Copyright © 2010 Pearson Education, Inc. Passive Processes • What determines whether or not a substance can passively permeate a membrane? 1. Lipid solubility of substance 2. Channels of appropriate size 3. Carrier proteins PLAY Animation: Membrane Permeability Copyright © 2010 Pearson Education, Inc. Passive Processes • Simple diffusion • Carrier-mediated facilitated diffusion • Channel-mediated facilitated diffusion • Osmosis Copyright © 2010 Pearson Education, Inc. Passive Processes: Simple Diffusion • Nonpolar lipid-soluble (hydrophobic) substances diffuse directly through the phospholipid bilayer PLAY Animation: Diffusion Copyright © 2010 Pearson Education, Inc. Extracellular fluid Lipidsoluble solutes Cytoplasm (a) Simple diffusion of fat-soluble molecules directly through the phospholipid bilayer Copyright © 2010 Pearson Education, Inc. Figure 3.7a Passive Processes: Facilitated Diffusion • Certain lipophobic molecules (e.g., glucose, amino acids, and ions) use carrier proteins or channel proteins, both of which: • Exhibit specificity (selectivity) • Are saturable; rate is determined by number of carriers or channels • Can be regulated in terms of activity and quantity Copyright © 2010 Pearson Education, Inc. Facilitated Diffusion Using Carrier Proteins • Transmembrane integral proteins transport specific polar molecules (e.g., sugars and amino acids) • Binding of substrate causes shape change in carrier Copyright © 2010 Pearson Education, Inc. Lipid-insoluble solutes (such as sugars or amino acids) (b) Carrier-mediated facilitated diffusion via a protein carrier specific for one chemical; binding of substrate causes shape change in transport protein Copyright © 2010 Pearson Education, Inc. Figure 3.7b Facilitated Diffusion Using Channel Proteins • Aqueous channels formed by transmembrane proteins selectively transport ions or water • Two types: • Leakage channels • Always open • Gated channels • Controlled by chemical or electrical signals Copyright © 2010 Pearson Education, Inc. Small lipidinsoluble solutes (c) Channel-mediated facilitated diffusion through a channel protein; mostly ions selected on basis of size and charge Copyright © 2010 Pearson Education, Inc. Figure 3.7c Passive Processes: Osmosis • Movement of solvent (water) across a selectively permeable membrane • Water diffuses through plasma membranes: • Through the lipid bilayer • Through water channels called aquaporins (AQPs) Copyright © 2010 Pearson Education, Inc. Water molecules Lipid billayer Aquaporin (d) Osmosis, diffusion of a solvent such as water through a specific channel protein (aquaporin) or through the lipid bilayer Copyright © 2010 Pearson Education, Inc. Figure 3.7d Passive Processes: Osmosis • Water concentration is determined by solute concentration because solute particles displace water molecules • Osmolarity: The measure of total concentration of solute particles • When solutions of different osmolarity are separated by a membrane, osmosis occurs until equilibrium is reached Copyright © 2010 Pearson Education, Inc. (a) Membrane permeable to both solutes and water Solute and water molecules move down their concentration gradients in opposite directions. Fluid volume remains the same in both compartments. Left compartment: Solution with lower osmolarity Right compartment: Solution with greater osmolarity Both solutions have the same osmolarity: volume unchanged H2O Solute Membrane Copyright © 2010 Pearson Education, Inc. Solute molecules (sugar) Figure 3.8a (b) Membrane permeable to water, impermeable to solutes Solute molecules are prevented from moving but water moves by osmosis. Volume increases in the compartment with the higher osmolarity. Left compartment Right compartment Both solutions have identical osmolarity, but volume of the solution on the right is greater because only water is free to move H2O Membrane Copyright © 2010 Pearson Education, Inc. Solute molecules (sugar) Figure 3.8b Importance of Osmosis • When osmosis occurs, water enters or leaves a cell • Change in cell volume disrupts cell function PLAY Animation: Osmosis Copyright © 2010 Pearson Education, Inc. Tonicity • Tonicity: The ability of a solution to cause a cell to shrink or swell • Isotonic: A solution with the same solute concentration as that of the cytosol • Hypertonic: A solution having greater solute concentration than that of the cytosol • Hypotonic: A solution having lesser solute concentration than that of the cytosol Copyright © 2010 Pearson Education, Inc. (a) Isotonic solutions Cells retain their normal size and shape in isotonic solutions (same solute/water concentration as inside cells; water moves in and out). Copyright © 2010 Pearson Education, Inc. (b) Hypertonic solutions Cells lose water by osmosis and shrink in a hypertonic solution (contains a higher concentration of solutes than are present inside the cells). (c) Hypotonic solutions Cells take on water by osmosis until they become bloated and burst (lyse) in a hypotonic solution (contains a lower concentration of solutes than are present in cells). Figure 3.9 Summary of Passive Processes Process Simple diffusion Facilitated diffusion Osmosis Energy Source Kinetic energy Kinetic energy Kinetic energy • Also see Table 3.1 Copyright © 2010 Pearson Education, Inc. Example Movement of O2 through phospholipid bilayer Movement of glucose into cells Movement of H2O through phospholipid bilayer or AQPs