* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Macromolecules 2016
Signal transduction wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Phosphorylation wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein structure prediction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
List of types of proteins wikipedia , lookup
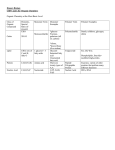
Macromolecules Biology Intro Video • https://vimeo.com/83005599 The Importance of CHNOPS • CARBON – Major structural atom in all organic molecules. – Key component in photosynthesis, returned back to the environment through cellular respiration, and decomposition. – CO is the major nonliving source of carbon in the atmosphere. 2 We will talk more about the biogeochemical cycles a little later! Hydrogen - Major component of all organic molecules. - Most common atom in the Universe. - Enters biological systems largely bonded to oxygen in water. - Returned to the environment by decomposition and water release. Nitrogen • Found in all proteins and nucleic acids. • Major nonliving source is N2 in the atmosphere. • Makes its way into the food chain via nitrogen fixing bacteria, which convert it into a usable form of N2 that can be used by producers and passed on to consumers in the food chain. • Returned back to the environment through decomposition and denitrifying bacteria (convert nitrates in the soil into atmospheric nitrogen). Oxygen • Found in most organic molecules. • Oxygen is in our atmosphere, as well as in our water. • Incorporated into the food chain through cellular respiration and returned back to the environment through photosynthesis. Phosphorus • Found in all nucleic acids • Used quickly to store and release free energy in cells. • Decomposition returns it back to the environment. Sulfur • Found in all proteins. • Major nonliving source is found in rocks. • Weathering releases it back into the soil, where producers absorb it and pass it through the food chain. • Decomposition returns it back to the environment. The building blocks of life! All contain the element carbon! Also known as biomolecules, carbon based molecules, and macromolecules Macromolecules Unique atomic structure because it has four unpaired electrons on the outer energy level, and can form covalent bonds with up to four other atoms!!!!!! There are four! 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Video • https://www.youtube.com/watch?v=YO244P1 e9QM Molecular Shape Ring Branched Straight Monomer / Polymer Polymer= molecule that contains many Monomers bonded together. Monomer= small molecular subunit How many monomers are above? • How do we make a polymer from a monomer? _________________________________________ • How do we break down a polymer?__________________________________ • ________________________________: a chemical process where two smaller molecules are combined to make a larger molecule. Water is released and energy is stored in the newly formed chemical bonds. • _______________________________: A chemical process where a large molecule is broken down into smaller molecules. Water is required and energy is released. Digestion is a series of hydrolytic reactions. Use the diagram below to help you answer these questions! Answers • 1. How do we make a polymer from a monomer? Dehydration synthesis / take water away. • 2. How do we break down a polymer? Hydrolysis / add water • 3. Dehydration Synthesis: a chemical process where two smaller molecules are combined to make a larger molecule. Water is released and energy is stored in the newly formed chemical bonds. • 4. Hydrolysis: A chemical process where a large molecule is broken down into smaller molecules. Water is required and energy is released. Digestion is a series of hydrolytic reactions. Macromolecule Chart Macromolecule Carbohydrates Lipids Proteins Nucleic Acids Basic Formula & Functional Group Monomer CHO 1:2:1 -OH (hydroxyl) Glucose Galactose Fructose Ribose Deoxyribose CHO 1:2: very few -CH3 (methyl) - OH CHONS No ratio Triglyceride (Glycerol + 3 fatty acids) NH2 (amino) COOH (carboxyl) CHONP PO4 No true monomer. Amino Acids = also known as “the building blocks of proteins.” Nucleotide: made up of a 5 carbon sugar, phosphate, and a nitrogenous base (A-T, C-G) Sub-groups and Polymers Examples Monosaccharides Glucose, Functions / Uses Main source of energy = simple sugars Disaccharides= double sugars galactose, fructose Sucrose, lactose Store energy Polysaccharides= Starch- plants many sugars GlycogenProvide structure animals Cellulose- plants Chitin- insects Carbohydrates Saturated= all single bonds, full of Butter H Long term energy storage Soft Margarine Monounsaturated= One double bond Olive Oil Polyunsaturated= two or more double bonds Cell Membrane Phospholipids (phosphate replaces a fatty acid) Steroids (4 fused rings) Cholesterol Testosterone Progesterone Waxes (-OH replaces a fatty acid) Beeswax (paraffin) Dipeptide= two amino acids Enzymes Polypeptide= many amino acids Muscles Protect the cell: selects what can enter and leave the cell. Chemical messengers Skin Some Hormones Repel water (leaves) Storage Signal Structural Contractile Defensive Enzyme Transport Receptor DNA Genes Stores and transmits genetic information. RNA mRNA, tRNA, rRNA Protein synthesis ATP, ADP, AMP Main source of energy for cells Practice identifying the macromolecule! Taking Volunteers! Explain how monomers are related to polymers. Match the Monomer on the left to the macromolecules on the right. Fatty acids and glycerol _________ A. Protein Monosaccharide _________ B. Lipid Nucleotide _________ C. Nucleic acid Amino acid _________ D. Carbohydrate Match the Polymer on the left to the macromolecules on the right. DNA _________ A. Protein Enzyme _________ B. Lipid Triglyceride _________ C. Nucleic acid Polysaccharide _________ D. Carbohydrate Match the Monomer on the left to the Polymer on the right. Fatty acids and glycerol _________ A. Polysaccharide Monosaccharide _________ B. RNA Nucleotide _________ C. Enzyme Amino acid _________ D. Phospholipid Match the Monomer on the left to the Polymer on the right. Fatty acids and glycerol _________ A. Enzyme Glucose _________ B. Triglyceride Nucleotide _________ C. Starch Amino acid _________ D. DNA Match the Monomer on the left to the Polymer on the right. Amino acid _________ A. Glycogen Nucleotide _________ B. Phospholipid Monosaccharide _________ C. Protein Fatty acids and glycerol _________ D. DNA Match the Polymer on the left to the macromolecules on the right. Cholesterol _________ A. Protein Enzyme _________ B. Nucleic Acid RNA _________ C. Carbohydrate Cellulose _________ D. Lipid How do we break down a polymer and turn it into a monomer? • Eat pasta (starch)-> There is a protein (enzyme) in your spit that breaks the pasta down -> glucose • Let’s try it! “The Saltine Cracker Experiment” – 1. Chew the cracker, DON’T SWALLOW IT – 2. Put another crack in, chew it, don’t swallow it, just chew. – 3. You should taste the cracker getting sweeter= glucose. What if glucose is needed now? • We make a polymer called glycogen (similar to starch, but only found in animals), which are repeating units, or monomers of glucose with lots of branches. Glycogen curls around and makes a BIG globby molecule. • Globby and branched= sticks out all over the place. • Enzymes attach to the ends, and break down the glycogen into glucose= ENERGY • Where is glycogen found, and where do you need it the most? Liver and Muscles! Phospholipids • Form the bilayer of the cell membrane. • First line of defense for the cell. • One glycerol, two fatty acids, and a phosphate. • Hydrophobic tails- made up of fatty acids, and are afraid of water (nonpolar). • Hydrophilic heads- made up of glycerol and phosphate, and love water (polar). Functions of Proteins Function Catalyzing Enzymes Description •Activate metabolic reactions, speed up rates of reactions. •Lowers activation energy-> the amount of energy needed to get a reaction started. •On-going / never stop. •Need certain factors in order to work properly-> pH, temperature, enzyme concentration, substrate concentration, and the presence of inhibitors. •Ex- Human enzymes work best at 98.6, above 104 they fall apart. Defensive Proteins •Basis of the bodies endocrine and immune systems. They attack invading microbes and cancer cells. •Ex- antibodies attack viruses and bacteria •Ex- fibrinogen = protein that causes your blood to clot Storage Proteins Bind with iron and calcium to provide nourishment for an organism. Function Description Transport Proteins •Allow larger molecules to move in and out of cells. Ex- Hemoglobin= carries oxygen Ex- Myoglobin= carries oxygen to muscles Support Proteins •Provide structural support and protection. •Ex- Keratin in your hair, skin, and nails •Ex- Fibrin- allows your blood to clot •Ex- Collagen and elastin- major components of connective tissue Motion Proteins •Such as myosin and actin, cause muscles to contract or change shape. Messenger Proteins •Allow different cells to communicate Ex- Hormones- regulate body functions Ex- Insulin- regulates glucose levels Ex- Vasopressin- tells your kidneys to reabsorb water • Deoxyribonucleic Acid • Double stranded, twisted ladder, double helix • Sugar= deoxyribose • Location= Nucleus only • Function= carries and transfers genetic info. • Processes= DNA Replication • Base Pairs= – A-T (Adenine – Thymine) – C-G (Cytosine- Guanine) – Known as Chargaff’s Rule • • • • • Ribonucleic Acid Single Stranded Sugar= Ribose Location= Nucleus and cytoplasm Function= carries and transfers genetic information and PROTEIN SYNTHESIS (the process by which the genetic code puts together proteins in the cell). • Processes: Transcription and Translation • Base Pairs= – A-U (Adenine – Uracil) – C-G (Cytosine to Guanine) Nucleic Acids Make sure to label all of the parts! DNA RNA Questions • 1. What is the monomer of a nucleic acid made up of? • 2. What type of bond holds together the nitrogenous bases? • 3. What type of bond holds together the sugars and phosphates? • 4. Which base pairs match up in DNA? • 5. Which base pairs match up in RNA? • 6. In RNA, thymine is replaced with ___________. • 1. Nucleotide: sugar, phosphate, and a nitrogenous base. • 2. Hydrogen bonds • 3. Covalent bonds • 4. A-T and C-G • 5. A-U and C-G • 6. Uracil Videos • https://www.youtube.com/watch?v=o_6JXLYS-k Four Macromolecules / Carbon Based Molecules 1.________________________________ 2.______________________________ 3.________________________________ 4.______________________________ Directions: Using the four macromolecules above, write which one is represented by the description. Put a C for carbohydrate, P for protein, L for lipid, and NA for nucleic acids. Stores and transmits genetic information.________ Makes Enzymes ________________ Insulin ______________ Sucrose ______________ Saturated ________________ Fatty Acids _____________ Glucose ______________ Antibodies _______________ Enzyme Substrate Complex _____________ Phospholipid Bilayer ________________ Contains nitrogenous bases __________ Amino acids ___________________ Monosaccharides _____________ Main component of the cell membrane ___________ The only one that contains phosphorus (sugar, phosphate, nitrogenous base) _______________ Glycerol _________________ Collagen _________________ Polyunsaturated ________________ Long term energy storage _______________ Main source of energy _____________ Cholesterol ________________ Hemoglobin _____________ Disaccharides _______________ Starches __________________ ATP___________________ Unsaturated fats ________________ Deoxyribonucleic Acid_________________ Ribonucleic Acid _________________ Steroids _________________ Lactose __________________ Ends in “ose” __________________ Olive oil __________________ Cellulose _________________ Triglycerides _________________ Has an “R” group _________________ Monomers are nucleotides _________________ Hormones ____________________ Let’s look at the molecular structures a little closer.