* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Pre-lab: Complete parts I—IV prior to conducting the laboratory.
Tissue engineering wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
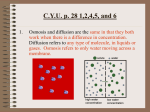
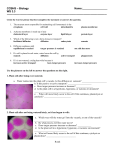
Instructions for AP Biology Investigation #4--Diffusion and Osmosis Pre-lab: Complete parts I—IV prior to conducting the laboratory. I. Title: Do not develop a title until you have completed the Pre-lab. Then, develop a title for this laboratory. II. Objectives: See Lab handout and rewrite the objectives into your pre-lab write-up. III. Pre-lab Questions: (pg S54-S55) 1. 2. 3. 4. 5. What is kinetic energy, and how does it differ from potential energy? What environmental factors affect kinetic energy and diffusion? How do these factors alter diffusion rates? Why are gradients important in diffusion and osmosis? What is the explanation for the fact that most cells are small and have cell membranes with many convolutions? 6. Will water move into or out of a plant cell if the cell has a higher water potential than the surrounding environment? 7. What would happen if you applied saltwater to a plant? 8. How does a plant cell control its internal (turgor) pressure? IV. Methods: Read the procedures carefully for each activity. You may write a 1-2 sentence summary for each part of the lab. V. Data: Procedure 2: A. Guiding Questions: 1. Why is it important for an IV solution to have salts in it? 2. What would happen if you were given pure water in an IV? 3. How would you determine the best concentration of solutes to give a patient in need 4. of fluids before you introduced the fluids into the patient’s body? 5. How can you use weights of the filled cell models to determine the rate and direction of diffusion? What would be an appropriate control for the procedure you just described? 6. Suppose you could test other things besides weights of the dialysis tubes. How could you determine the rates and directions of diffusion of water, sucrose, NaCl, glucose, and ovalbumin? 7. Will protein diffuse? Will it affect the rate of diffusion of other molecules? B. Draw: your set up and underneath each drawing make your prediction about where the water will diffuse. C. Data Table: Create a data table with your information from Step 3 and Step 4. D. Conclusion Questions: 1. Which pair(s) that you tested did not have a change in weight? How can you explain this? 2. If you compared 1 M solutions, was a 1 M NaCl solution more or less hypertonic than a 1 M sucrose solution? What is your evidence? What about 1 M NaCl and 1M glucose and 1 M sucrose? 3. Does the protein solution have a high molarity? What is evidence for your conclusion? 4. How could you test for the diffusion of glucose? 5. Based on what you learned from your experiment, how could you determine the solute concentration inside a living cell? E. Design and Conducting Your Investigation: Use the same outline above for this section. Draw out your set-up, make predictions and create a data table. Procedure 3: Observing Osmosis in Living Cells A. Guiding Questions 1. What would happen if you applied saltwater to the roots of a plant? Why? 2. What are two different ways a plant could control turgor pressure, a name for internal water potential within its cells? Is this a sufficient definition for turgor pressure? 3. Will water move into or out of a plant cell if the cell has a higher water potential than its surrounding environment? B. Draw cells -Draw cells under light microscope at 400X magnification. Label the cell membrane and cell wall. Label any other structures you are able to see. 1. Where is the cell membrane in relation to the cell wall? Can you see the two structures easily? Why or why not? 2. What parts of the cell that you see control the water concentration inside the cell? - Expose cells to one of the solutions from Procedure 2. Look at these cells under the microscope and draw what you see. Label any of the structures you are able to see. 3. What changes do you expect to see when the cells are exposed to the solutions? 4. How will you know if a particular treatment is increasing turgor pressure? If it is reducing turgor pressure? 5. How could you determine which solution is isotonic to the cells? C. Designing and Conducting Your Investigation 1. How can you measure the plant pieces to determine the rate of osmosis? 2. How would you calculate the water potential in the cells? 3. Which solution had a water potential equal to that of the plant cells? How do you know? 4. Was the water potential in the different plants the same? 5. How does this compare to your previous determinations in the Elodea cells? 6. What would your results be if the potato were placed in a dry area for several days before your experiment? 7. When potatoes are in the ground, do they swell with water when it rains? If not, how do you explain that, and if so, what would be the advantage or disadvantage? Use the following procedures to guide your experiment design PART VI: Conclusion Part VII: Theme Correlation Explain how this lab addresses at least one of the Big Ideas of AP Biology.