* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Online Counseling Resource YCMOU ELearning Drive…
Survey
Document related concepts
Genetic code wikipedia , lookup
Signal transduction wikipedia , lookup
Paracrine signalling wikipedia , lookup
Gene expression wikipedia , lookup
Point mutation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Expression vector wikipedia , lookup
Metalloprotein wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Biochemistry wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Homology modeling wikipedia , lookup
Interactome wikipedia , lookup
Western blot wikipedia , lookup
Protein purification wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Transcript
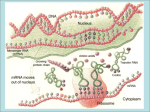
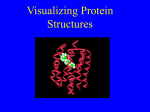
Online Counseling Resource YCMOU ELearning Drive… School of Architecture, Science and Technology Yashwantrao Chavan Maharashtra Open University, Nashik – 422222, India SEP-SBI074-CP3-04 Introduction Programmes and Courses SEP –SBI074-CP3-U03 School of Science and Technology, Online Counseling Resource… Credits Academic Inputs by Sonali Alkari Faculty YCMOU Nagpur Centre, Faculty LAD college P.G. D of Biotechnology Research officer Ankur Seeds Pvt Ltd [email protected] [email protected] © 2008, YCMOU. All Rights Reserved. 3 School of Science and Technology, Online Counseling Resource… How to Use This Resource Counselor at each study center should use this presentation to deliver lecture of 40-60 minutes during Face-To-Face counseling. Discussion about students difficulties or tutorial with assignments should follow the lecture for about 40-60 minutes. Handouts (with 6 slides on each A4 size page) of this presentation should be provided to each student. Each student should discuss on the discussion forum all the terms which could not be understood. This will improve his writing skills and enhance knowledge level about topics, which shall be immensely useful for end exam. Appear several times, for all the Self-Tests, available for this course. Student can use handouts for last minutes preparation just before end exam. © 2008, YCMOU. All Rights Reserved. 4 School of Science and Technology, Online Counseling Resource… Learning Objectives After studying this module, you should be able to : Describe protein folding Discuss mechanism of protein folding Describe techniques involved in studying protein folding. © 2008, YCMOU. All Rights Reserved. 5 School of Science and Technology, Online Counseling Resource… Introduction: Protein Folding Each protein begins as a polypeptide, translated from a sequence of mRNA as a linear chain of amino acids. This polypeptide lacks any developed threedimensional structure. However each amino acid in the chain can be thought of having certain 'gross' chemical features. These may be hydrophobic, hydrophilic, or electrically charged. These interact with each other and their surroundings in the cell to produce a well-defined, three dimensional shape, the folded protein., known as the native state. The resulting three-dimensional structure is determined by the sequence of the amino acids. © 2008, YCMOU. All Rights Reserved. 6 School of Science and Technology, Online Counseling Resource… Folding and Amino Acid Sequence-1 The mechanism of protein folding is not completely understood. The amino-acid sequence (or primary structure) of a protein predisposes it towards its native conformation or conformations. It will fold spontaneously during or after synthesis. While these macromolecules may be regarded as "folding themselves", the mechanism depends equally on the characteristics of the cytosol, including the nature of the primary solvent (water or lipid), the concentration of salts, the temperature, and molecular chaperones. © 2008, YCMOU. All Rights Reserved. 7 School of Science and Technology, Online Counseling Resource… Folding and Amino Acid Sequence-2 Most folded proteins have a hydrophobic core in which side chain packing stabilizes the folded state, and charged or polar side chains on the solvent-exposed surface where they interact with surrounding water molecules. It is generally accepted that minimizing the number of hydrophobic side chains exposed to water is the principal driving force behind the folding process. Although a recent theory has been proposed which reassesses the contributions made by hydrogen bonding. © 2008, YCMOU. All Rights Reserved. 8 School of Science and Technology, Online Counseling Resource… Folding and Amino Acid Sequence-3 The process of folding in vivo often begins cotranslationally, so that the N-terminus of the protein begins to fold while the C-terminal portion of the protein is still being synthesized by the ribosome. Specialized proteins called chaperones assist in the folding of other proteins. A well studied example is the bacterial GroEL system, which assists in the folding of globular proteins. In eukaryotic organisms chaperones are known as heat shock proteins. © 2008, YCMOU. All Rights Reserved. 9 School of Science and Technology, Online Counseling Resource… Mechanism of Protein Folding:1 Scientists have been able to study many identical molecules folding together en masse. At the coarsest level, it appears that in transitioning to the native state, a given amino acid sequence takes on roughly the same route and proceeds through roughly the same intermediates and transition states. Often folding involves first the establishment of regular secondary and supersecondary structures, particularly alpha helices and beta sheets, and afterwards tertiary structure. Formation of quaternary structure usually involves the "assembly" or "coassembly" of subunits that have already folded. © 2008, YCMOU. All Rights Reserved. 10 School of Science and Technology, Online Counseling Resource… Mechanism of Protein Folding:2 The regular alpha helix and beta sheet structures fold rapidly because they are stabilized by intramolecular hydrogen bonds, as was first characterized by Linus Pauling. Protein folding may involve covalent bonding in the form of disulfide bridges formed between two cysteine residues or the formation of metal clusters. Shortly before settling into their more energetically favourable native conformation, molecules may pass through an intermediate "molten globule" state. © 2008, YCMOU. All Rights Reserved. 11 School of Science and Technology, Online Counseling Resource… Protein Folding © 2008, YCMOU. All Rights Reserved. 12 School of Science and Technology, Online Counseling Resource… Facts of Protein Folding-1 The essential fact of folding, however, is that the amino acid sequence of each protein contains the information that specifies both the native structure and the pathway. This is not to say that identical amino acid sequences always fold similarly. Conformations differ based on environmental factors as well; similar proteins fold differently based on where they are found. Folding is a spontaneous process independent of energy inputs from nucleoside triphosphates. © 2008, YCMOU. All Rights Reserved. 13 School of Science and Technology, Online Counseling Resource… Facts of Protein Folding-2 The passage of the folded state is mainly guided by hydrophobic interactions, formation of intramolecular hydrogen bonds, and van der Waals forces, and it is opposed by conformational entropy, which must be overcome by extrinsic factors such as chaperones. Some proteins never fold in cells at all except with the assistance of chaperone molecules, which either isolate individual proteins so that their folding is not interrupted by interactions with other proteins or help to unfold misfolded proteins, giving them a second chance to refold properly. © 2008, YCMOU. All Rights Reserved. 14 School of Science and Technology, Online Counseling Resource… Disruption of the Native State-1 In certain solutions and under some conditions proteins will not fold into their biochemically functional forms. Temperatures above (and sometimes those below) the range that cells tend to live in will cause proteins to unfold or "denature" (this is why boiling makes the white of an egg opaque). High concentrations of solutes, extremes of pH, mechanical forces, and the presence of chemical denaturants can do the same. A fully denatured protein lacks both tertiary and secondary structure, and exists as a so-called random coil. © 2008, YCMOU. All Rights Reserved. 15 School of Science and Technology, Online Counseling Resource… Disruption of the Native State-2 Under certain conditions some proteins can refold; however, in many cases denaturation is irreversible. Cells sometimes protect their proteins against the denaturing influence of heat with enzymes known as chaperones or heat shock proteins, which assist other proteins both in folding and in remaining folded. This function is crucial to prevent the risk of precipitation into insoluble amorphous aggregates. © 2008, YCMOU. All Rights Reserved. 16 School of Science and Technology, Online Counseling Resource… Techniques for Studying Protein Folding Modern studies of folding with high time resolution. Energy landscape theory of protein folding. Computational prediction of protein tertiary structure. Techniques for determination of protein structure © 2008, YCMOU. All Rights Reserved. 17 School of Science and Technology, Online Counseling Resource… Modern Studies of Folding with High Time Resolution The study of protein folding has been greatly advanced in recent years by the development of fast, time-resolved techniques. These are experimental methods for rapidly triggering the folding of a sample of unfolded protein, and then observing the resulting dynamics. Fast techniques in widespread use include ultrafast mixing of solutions, photochemical methods, and laser temperature jump spectroscopy. © 2008, YCMOU. All Rights Reserved. 18 School of Science and Technology, Online Counseling Resource… Energy Landscape Theory of Protein Folding-1 The protein folding phenomenon was largely an experimental endeavor until the formulation of energy landscape theory by Joseph Bryngelson and Peter Wolynes in the late 1980s and early 1990s. This approach introduced the principle of minimal frustration, which asserts that evolution has selected the amino acid sequences of natural proteins so that interactions between side chains largely favor the molecule's acquisition of the folded state. Interactions that do not favor folding are selected against, although some residual frustration is expected to exist. © 2008, YCMOU. All Rights Reserved. 19 School of Science and Technology, Online Counseling Resource… Energy Landscape Theory of Protein Folding-2 A consequence of these evolutionarily selected sequences is that proteins are generally thought to have globally "funneled energy landscapes" (coined by José Onuchic) that are largely directed towards the native state. This "folding funnel" landscape allows the protein to fold to the native state through any of a large number of pathways and intermediates, rather than being restricted to a single mechanism. The theory is supported by both computational simulations of model proteins and numerous experimental studies, and it has been used to improve methods for protein structure prediction and design. © 2008, YCMOU. All Rights Reserved. 20 School of Science and Technology, Online Counseling Resource… Computational Prediction of Protein Tertiary Structure1 De novo or ab initio techniques for computational protein structure prediction is related to, but strictly distinct from, studies involving protein folding. Molecular Dynamics (MD) is an important tool for studying protein folding and dynamics in silico. Because of computational cost, ab initio MD folding simulations with explicit water are limited to peptides and very small proteins. MD simulations of larger proteins remain restricted to dynamics of the experimental structure or its high-temperature unfolding. © 2008, YCMOU. All Rights Reserved. 21 School of Science and Technology, Online Counseling Resource… Computational Prediction of Protein Tertiary Structure-2 In order to simulate long time folding processes (beyond about 1 microsecond), like folding of small-size proteins (about 50 residues) or larger, some approximations or simplifications in protein models need to be introduced. An approach using reduced protein representation (pseudo-atoms representing groups of atoms are defined) and statistical potential is not only useful in protein structure prediction, but is also capable of reproducing the folding pathways. © 2008, YCMOU. All Rights Reserved. 22 School of Science and Technology, Online Counseling Resource… Techniques for determination of Protein Structure The determination of the folded structure of a protein is a lengthy and complicated process, involving methods like X-ray crystallography and NMR. One of the major areas of interest is the prediction of native structure from amino-acid sequences alone using bioinformatics and computational simulation methods. © 2008, YCMOU. All Rights Reserved. 23 School of Science and Technology, Online Counseling Resource… Incorrect Protein Folding & Neurodegenerative Disease Misfolded proteins are responsible for prionrelated illnesses such as Creutzfeldt-Jakob disease, bovine spongiform encephalopathy (mad cow disease), amyloid-related illnesses such as Alzheimer's Disease, and a number of other forms of proteopathy such as cystic fibrosis. These diseases are associated with the multimerization of misfolded proteins into insoluble, extracellular aggregates and/or intracellular inclusions; it is not clear whether the plaques are the cause or merely a symptom of illness. © 2008, YCMOU. All Rights Reserved. 24 School of Science and Technology, Online Counseling Resource… What You Learn… You have learnt : The Interaction of hydrophobic, hydrophilic, or electrical charges of protein sequences with each other and their surroundings in the cell to produce a well-defined, three dimensional shape, the folded protein. The mechanism of protein folding is not completely understood. Specialized proteins called chaperones assist in the folding of other proteins. Folding is a spontaneous process independent of energy inputs from nucleoside triphosphates. "folding funnel" landscape allows the protein to fold to the native state through any of a large number of pathways and intermediates, rather than being restricted to a single mechanism. © 2008, YCMOU. All Rights Reserved. 25 School of Science and Technology, Online Counseling Resource… Critical Thinking Questions 1. Describe in details what is protein folding. 2. Describe the folding mechanism of protein 3. State the facts of protein folding. 4. Describe the various techniques involved in protein folding . © 2008, YCMOU. All Rights Reserved. 26 School of Science and Technology, Online Counseling Resource… Hints For Critical Thinking Question 1. The Interaction of hydrophobic, hydrophilic, electrical charges of protein sequences or 2. Folding involves first the establishment of regular secondary and supersecondary structures, particularly alpha helices and beta sheets, and afterwards tertiary structure. 3. Folding is a spontaneous process independent of energy inputs from nucleoside triphosphates. 4. High time resolution, Energy landscape theory, Computational prediction and Techniques for determination of protein structure. © 2008, YCMOU. All Rights Reserved. 27 School of Science and Technology, Online Counseling Resource… Study Tips:1 Book1 Title: Molecular Cell Biology Author: Harvey Lodish, David Baltimore Publisher:Publishers: W. H. Freeman and Company Book2 Title: Principles of Biochemistry Author: AlbertL Lehninger Publisher:CBS Publishers & Distributors © 2008, YCMOU. All Rights Reserved. 28 School of Science and Technology, Online Counseling Resource… Study Tips:2 Book3 Title: Biochemistry Author: Lubert stryer Publishers: Freeman International Book4 Title: Biochemistry Author: Keshav Trehan Publishers: Wiley Eastern © 2008, YCMOU. All Rights Reserved. 29 School of Science and Technology, Online Counseling Resource… Study Tips www.en.wikipedia.org Microsoft Encarta Encyclopedia http://en.wikipedia.org/wiki/ Wikipedia the free encyclopedia © 2008, YCMOU. All Rights Reserved. 30 School of Science and Technology, Online Counseling Resource… End of the Presentation Thank You © 2008, YCMOU. All Rights Reserved. 31