* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development from Neural Crest Cells
Multielectrode array wikipedia , lookup
Metastability in the brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Nervous system network models wikipedia , lookup
Artificial neural network wikipedia , lookup
Neuroanatomy wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Subventricular zone wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Recurrent neural network wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Neural engineering wikipedia , lookup
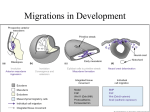
Development from Neural Crest Cells Neural Crest Cells as Precursor Cells for Multiple Lineages • Neural crest cells migrate extensively to generate multiple differentiated cell types. • neurons and glial cells of the sensory, sympathetic and parasympathetic nervous system • epinephrine-producing (medulla) cells of the adrenal gland • pigment-containing cells of the epidermis • majority of the skeletal and connective tissue components in the head (cf. for trunk & posterior somites) • The fate of the neural crest cells depends, to a large degree, on where they migrate to and settle. Birth of Neural Crest Cells (NCCs) • Quail neural plate graft into chick non-neural ectoderm induces a second neural crest cell population. (contributions from both neural and epidermal ectoderms) • High expression of BMPs initially in the neural ectoderm (neural plate) later in dorsal neural tube. • Ventral NT : NC/FP Shh Noggin BMP inhibition • High expression of Wnt6 initially in epidermal ectoderm later in surface ectoderm • Cells under the influences from both high levels of BMPs and Wnt6 give rise to neural crest cells. • These presumptive neural crest cells can be identified by their characteristic expression of two marker genes such as Slug and FoxD3. • FoxD3 : NCC fate specification; FoxD3 inhibition No NCC; Forced FoxD3 expression Ectopic NCC formation • Slug : NCC migration out of epithelium Four Main Domains of Neural Crest 1) Cranial (Cephalic) Neural Crest A. migrate dorsolaterally to produce craniofacial mesenchyme cartilage, bone, neurons, glia, and connective tissues in the face B. enter anterior pharyngeal arches and pouches thymic cells, odontoblasts (tooth primordia), middle ear & jaw bones 2) Trunk Neural Crest (S8-27) A. migrate ventrolaterally and remain in the anterior half of each sclerotome dorsal root ganglia (sensory neurons) B. migrate more ventrally sympathetic ganglia, adrenal medulla, and nerve clusters around aorta C. migrate dorsolaterally into the ectoderm melanocytes (pigment synthesizing cells) 3) Vagal (S1-7) and Sacral (S28 to posterior) Neural Crest parasympathetic enteric ganglia of the gut (colon) (cf. neurons related to the peristaltic bowel movement) 4) Cardiac (somite 1-3) Neural Crest (posterior to cranial NC) A. migrate into the third, fourth and sixth pharyngeal arches melanocytes, neurons, cartilage, and connective tissue B. entire muscular-connective tissue wall of the large arteries (arise from the heart) C. septum separating the pulmonary circulation from the aorta Migration Pathways of Trunk Neural Crest Cells 1) Ventral Pathway • Sensory neurons (dorsal root ganglia) • Sympathetic neurons • Adrenomedullary cells • Schwann cells • In birds and mammals, this migration is going through only anterior section of each sclerotome. 2) Dorsolateral Pathway • melanocytes : melanin-forming cells • migrate between epidermis and dermis layers • enter the ectoderm through small holes in the basal lamina HNK-1 Staining Neural Crest Cell Emigration from the Neural tube • The neural crest cells are originally part of an epithelium. • The NCCs undergo EMT (Epithelial-to-Mesenchymal Transformation), thus the NCCs can be detached from other cells. Then they migrate away from the epithelial sheet. • Wnts, FGFs, and BMPs together induce the expression of NCC specific genes such as Slug and RhoB. • RhoB : Cytoskeletal changes to promote migration (microfilament polymerization) • Slug Dissociates the intercellular tight-junctions to render cell movements. • Downregulation of N-cadherin expression at the time of migration • Reappearance of N-cadherin expression upon NCC aggregation after reaching to the final destination (e.g. to form dorsal root and sympathetic ganglia) HNK-1 : Red, RhoB : Green Overlapping Staining : Yellow Recognition of Surrounding Extracellular Matrices • ECMs such as fibronectin, laminin, tenascin, various collagens and proteoglycans promote migration of neural crest cells. • Integrin expression in NCCs upon migration (e.g. integrin α4β1) • When these integrin proteins are lacking, NCCs released from the neural tube become disoriented and often time undergo apoptosis. • Thrombospondin, expressed only in the anterior portion of the sclerotome, promotes NCC adhesion and migration by cooperating with fibronectin and laminin. • NCC migration impeding molecules - Ephrine proteins (Eph) expressed in the posterior section of each sclerotome - Ephrine receptor (Eph-R) expressed in NCCs - Eph binding to Eph-R activates tyrosine kinase activity of Eph-R in NCCs. - Then, Eph-R signaling interferes microfilament polymerization and more …. • This patterning also correlates with the actual structures of the neural crest derived peripheral nervous system such as dorsal root ganglia segmental positioning. • SCF (Stem Cell Factor) supports NCC proliferation for future melanocytes, protects apoptosis, and also functions as a chemotactic factor. (e.g. Forced expression of SCF in the chick footpad can unusually recruit melanocytes to this region.) The migration of neural crest cells can be regulated both by the ECMs and soluble signaling factors secreted at their potential destinations. Ephrine (+) region HNK-1 (+) cells negative correlation Motor neurons are emerged only from the anterior portion of each sclerotome FN Coating & Ephrin Strip Injection of Fluorescence-Labeled Neural Crest Cells Diverse Destinations Pluripotency of Neural Crest Cells • The vagal (neck) NCCs produce cholinergic neurons in parasympathetic ganglia. • The thoracic (trunk) NCCs produce adrenergic neurons in sympathetic ganglia. • But, when those NCCs are reciprocally transplanted, a new differentiation fate is determined based on their new locations. • NCCs express enzymes synthesizing for both acetylcholine and norepinephrine at pre-migratory stages. After migration, one of either enzyme is downregulated. • NCCs from the hindbrain region normally migrate into the eye and interact with the pigmented retina to become scleral cartilage. (i.e. no putative destinations to give rise to neural tissues) • But, when transplanted into the trunk region, they can be differentiated into sensory ganglion neurons, adrenomedullary cells, glia and Schwann cells. • Individual NCCs are pluripotent as they leave the crest. • Further differentiation of a neural crest cell depends on its eventual location. • BUT, some recent efforts have demonstrated that the initial NCC populations can be a mixture of different precursor cells. Gradual Determination of Neural Crest Cell fates Pluripotency of NCCs changes upon interactions with surrounding tissues during migration Signal Transduction Pathways Regulate the NCC Fate Specification The Fates of Neural Crest Cells Are Directed by the Tissue Environment Where They Settle • Ephrin signals inhibit early ventral migration, but later facilitate dorsolateral migration. Thus it seems critical for controlling the timing or onset of lateral migration of NCCs. • LIF (leukemia inhibition factor) from heart converts adrenergic neurons into cholinergic neurons. • BMP2 secreted by the heart, lungs, and dorsal aorta cholinergic neuron differentiation • GGF (glial growth factor; neuregulin) glial fates (cf. suppresses neuronal fates) • Wnt1 sensory neurons • Endothelin-3 adrenergic neuron • Endothelin-3 + Wnts melanocyte differentiation • Glucocorticoids adrenomedullary cell differentiation (chromaffin cells) (cf. suppresses neuronal fates) • NGF and FGF2 sympathetic neurons Cranial Neural Crest • Trunk NCCs melanocytes, neurons and glia • Cranial NCCs cartilage, bone, melanocytes, neurons and glial cells • Trunk NCCs can not give rise to cartilage tissues. • But, if trunk NCCs are placed into the head region, these NCC can normally differentiate into head cartilages and bones, which suggests that tissues around cranial NCCs provide competence factors for cranial NCCs to become cartilage or bone. Rhombomeres NCCs Pharyngeal Arches Various Cranial Structures • Rhombomere 1 and 2 first pharyngeal (mandibular) arch jaw, ear, frontonasal process • Rhombomere 4 second pharyngeal arch neck, middle ear • Rhombomere 6 third and fourth pharyngeal arches thymus, thyroid, parathyroid glands clavicle (collar bone) ; neck muscle attachment site • Rhombomere 3 and 5 no direct migration out of these rhombomeres NCC Specific β-Galactosidase Transgenic Mice NC Originated Precursor Cells Majority of Cranial Structures Vertebrate Skeletons Intramembranous Ossification Endochondral Ossification Mesenchymal Cells Mesenchymal Cells Chondrogenesis Skull Facial Bones Teeth Cartilage (Chondrocyte & ECMs) Somite (Sclerotome) Axial : Vertebrae, Ribs Lateral Plate Mesoderm Appendicular : Limbs Mesenchyme is made of loosely scattered cells that make up the majority of the undifferentiated tissue in the embryo. Intramembraneous Ossification periosteum NCC Mesenchyme Condensation Osteoblasts Osteocytes (Bone Cells) Capillaries CBFA1 Is Essential for Osteogenesis BMP 2, 4, 7 Mesenchymal Cells CBFA1 (Runx2) Expression Induction of Bone Specific Matrix Proteins such as Osteocalcin & Osteopontin Wildtype CBFA1 K.O. Cartilage Tissues Prefigure Entire Axial and Appendicular Skeletons Alcian Blue : Cartilage Specific Staining Reagent Endochondral Ossification & Longitudinal Bone Growth Proliferating Chondrocytes Highly Proliferative & Resistant to Apoptosis Responsible for Longitudinal Bone Growth Hypertrophic Chondrocytes Growth Arrested & Sensitive to Apoptosis Target Site for Osteogenesis & Vascularization Tooth Development Ectoderm Epithelium Enamel Knot Cusp Formation (Pre)Odontoblast Dentin Secreting Cells NCC Ectomesenchyme FGF8 Pax9 Mesenchyme Condensation Dental Papilla Mesoderm Enamel Knot Shh, FGF4, BMP 2, 4, 7 Jaw Epithelium Ameloblast Enamel Secreting Cells Cardiac Neural Crest • The heart originally forms in the neck region directly beneath the pharyngeal arches. • Cardiac neural crest : caudal region of the cranial neural crest • Cardiac neural crest cells give rise to the endothelium of the aortic arch arteries and the septum between the aorta and the pulmonary artery. • In mice, pax3 can be a cardiac neural crest marker. • Mutation in pax3 results in fewer cardiac neural crest cells and this condition causes persistent truncus arteriosus (the failure of the aorta and pulmonary artery to separate). • This mutation is also related to the congenital defects in thymus, thyroid, parathyroid glands. Cranial Placode • The anterior borders between the epidermal and neural ectoderm; local and transient thickenings of the ectoderm in the head and neck; Sensory apparatus (e.g. nose, ear, taste receptors, lens) • The otic placode, which develops into the sensory cells of the inner ear, is induced in the region of the ectoderm where the presumptive neural plate meets the presumptive epidermis. • FGF19 and Wnt8c synergistically induce the otic placode. • The epibranchial placodes form dorsally to where the pharyngeal pouches contact the epidermis to give rise to facial neurons and vagal nerves, etc. • Secondary NCCs from the placodes do not travel ventrally, rather they migrate dorsally to form glial cells and these glia form the tracks that guide neurons from the epibranchial placodes to the hindbrain. Therefore, it is very critical for hindbrain innervation.