* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 10 Outline: Alcohols
Survey
Document related concepts
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Stille reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
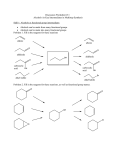
C341/Spring 2010 Chapter 10 Chapter 10 Outline: Alcohols Review Nomenclature, Structure & Physical Properties on your own 1. Acidity of Alcohols 2. Reactions of Alcohols 3. Reactions of Thiols You should do all the problems in the textbook, but these are some suggested homework problems: 10.15, 10.16, 10.18, 10.20, 10.21, 10.26 – 10.31, 10.35, 10.35 – 10.40, 10.42, 10.43, 10.45, 10-51 – 10.54. Page 1 of 23 C341/Spring 2010 Chapter 10 Roadmap for chapter 10: Page 2 of 23 C341/Spring 2010 Chapter 10 1. Acidity of Alcohols Alcohols have pKas in the range of 16-18. Only methanol has an acidity less than water (pKa 15.5 and will protonate water preferentially). In general, all alcohols will be less polar and less acidic than water. What happens when water is in solution with ethanol (pKa = 15.9)? Reaction of an alcohol with strong base, like sodium hydride, or Group I metals (Li, Na, K): • CH3OH + NaH → • 2 CH3OH + 2 Na → CH3ONa + H2 + H2 sodium methoxide 2 CH3ONa Page 3 of 23 C341/Spring 2010 Chapter 10 2. Reactions of Alcohols – how to make a good leaving group? All of these reactions you will learn in this chapters have one common theme – converting the –OH group into a good, stable leaving group!! What if we protonate the –OH group? Does that become a better leaving group? A strong, non-halide containing acid like H3PO4 or H2SO4 or TsOH is used to protonate an alcohol. = TsOH SO3H p‐toluene sulfonic acid Page 4 of 23 C341/Spring 2010 Chapter 10 a. ROH reaction with HCl, HBr & HI (converting a ROH →RX) 1o ROH react through an SN2 mechanism: 2o/3o ROH react through an SN1 mechanism: Page 5 of 23 C341/Spring 2010 b. Chapter 10 Reaction with PX3, X = Cl, Br (converting a ROH →RBr) Reaction Observations: o This reaction can only be used with 1o and 2o ROH only o Reaction always occurs with inversion of configuration. o What substitution mechanism does this support? Mechanism? Page 6 of 23 C341/Spring 2010 c. Chapter 10 Reaction with Thionyl Halide (SOX2, X = Cl, Br) (converting a ROH →RX) Reaction Observations: o This reaction can only be used with 1o and 2o ROH only o Reaction always occurs with inversion of configuration. o What substitution mechanism does this support? Mechanism? Page 7 of 23 C341/Spring 2010 Chapter 10 d. Formation of Tosylates to provide good leaving groups What is important about this? Sulfonate anions are weak bases, making them VERY GOOD leaving groups. Look at H2SO4 and consider its acidity: Since alcohols make poor leaving groups, reacting them with sulfonyl chlorides converts them into better leaving groups. Most common sulfonyl chloride reagents: O O H3C S Cl H3C S Cl O O TsCl (Para-toluene sulfonyl chloride) Page 8 of 23 MsCl (mesyl chloride) C341/Spring 2010 Chapter 10 How do these reactions work overall? It’s a two step reaction, (1) react the desired alcohol with the tosyl chloride without inversion, and (2) react it with the nucleophile leading to inversion of configuration: Page 9 of 23 C341/Spring 2010 Chapter 10 e. Acid-catalyzed Dehydration of Alcohols to Alkenes (β-elimination reaction) Compare to: E1 mechanism is observed for 2o and 3o ROHs: How do you think the mechanism differs for a 1o ROH? Page 10 of 23 C341/Spring 2010 Chapter 10 Provide the mechanism for the following transformation: Page 11 of 23 C341/Spring 2010 f. Chapter 10 Dehydration of ROH using POCl3 & Pyridine (converting a ROH →alkene) This mechanism is known to go through an E2 mechanism. Mechanism? Page 12 of 23 C341/Spring 2010 Chapter 10 g. Pinnacol Rearragenment Page 13 of 23 C341/Spring 2010 h. Chapter 10 Oxidation of Alcohols (no mechanism to know) • Primary alcohols are oxidized to either aldehydes or carboxylic acids, depending on the reagent employed: • Secondary alcohols are oxidized to ketones. • Tertiary alcohols cannot be oxidized – why? Common oxidizing reagents are: Page 14 of 23 C341/Spring 2010 o Chapter 10 1 ROH give different products depending on the reagent used: OH PCC K2Cr2O7 OH H2SO4, H2O 2o ROH give same products regardless of the reagent used: Provide the organic product for the following reactions. You do not need to balance the byproducts of the reaction. Page 15 of 23 C341/Spring 2010 Chapter 10 i. Reaction of glycols with periodic acid Page 16 of 23 C341/Spring 2010 Chapter 10 3. Reactions of Thiols L-Cysteine = Page 17 of 23 C341/Spring 2010 Chapter 10 Alcohol Reactions. Provide correct organic product(s) and the mechanism for the following reactions. If stereochemistry pertains, ensure it is clearly demonstrated. If there is more than one product, then circle the major product. OH H3PO4, heat Mechanism? OH POCl3 pyridine Mechanism? Page 18 of 23 C341/Spring 2010 Chapter 10 OH PBr3 Mechanism? OH HBr Mechanism? Page 19 of 23 C341/Spring 2010 Chapter 10 OH SOCl2 pyridine Mechanism? OH 1. TsCl, pyridine O 2. ONa Mechanism? OH 1. NaH 2. CH3CH2Br Mechanism? Page 20 of 23 C341/Spring 2010 Chapter 10 Provide viable steps to bring about the following transformation. Page 21 of 23 C341/Spring 2010 Chapter 10 Provide viable steps to bring about the following transformation. Page 22 of 23 C341/Spring 2010 Chapter 10 Provide viable steps to bring about the following transformation. Page 23 of 23