* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohol, Aldehydes and Acids
Survey
Document related concepts
Transcript
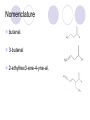
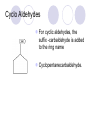
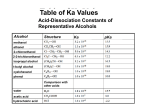
Alcohol, Aldehydes and Acids Some organic Functional Groups Alcohols An alcohol is any compound with an OH group (alcohol group) attached to single bonded hydrocarbons (alkanes). Ethanol Ethanol, when fermented from sugar, is the alcohol in beverages. It can also be made from ethene by the addition of water for nonbeverage use, like an additive to gassoline to make "gasahol." 2-Propanol (better known as isopropyl alcohol) is in (with some water) rubbing alcohol. It is also used in gasoline to prevent freezing of the gas line in automobiles by keeping excess moisture dissolved in the gasoline. Types of alcohols The mono hydroxyl alcohols can be classified : - primary alcohols - secondary alcohols - tertiary alcohols What about more than one alcohol group? ethylene glycol Aka: Ethanediol Diols Diols are named systematically as poly-alcohols, e.g. HOCH2CH2OH = 1,2ethanediol, so the same nomenclature rules as for alcohols apply. Diols Nomenclature: Diols are named systematically as poly-alcohols, e.g. HOCH2CH2OH = 1,2-ethanediol, so the same nomenclature rules as for alcohols apply. ethanediol 1,2-propanediol 1,3-propanediol cis-1,2-cyclohexanediol Alcohols Alcohols (R-OH) take the suffix "-ol" with an infix numerical bonding position: CH3CH2CH2OH is propan-1-ol. The suffixes -diol, -triol, -tetraol, etc., are used for multiple -OH groups: Ethylene glycol CH2OHCH2OH is ethane-1,2-diol. If higher precedence functional groups are present (see order of precedence, below), the prefix "hydroxy" is used with the bonding position: CH3CHOHCOOH is 2hydroxypropanoic acid. Aldehydes The aldehyde fucntional group is a -CHO which looks like this: Ends in -al Nomenclature butanal. 3-butenal 2-ethylhex3-ene-4-yne-al. Cyclo Aldehydes For cyclic aldehydes, the suffix -carbaldehyde is added to the ring name Cyclopentanecarbaldehyde. Carboxylic Acids The naming of carboxylic acids is fairly simple. You simply find the longest carbon chain which includes the carboxylic group. Use that as the stem for the name, cross off the -e on the ending of the alkane name and replace it with -oic acid Examples Pentanoic Acid Draw 2bromohexanoic acid